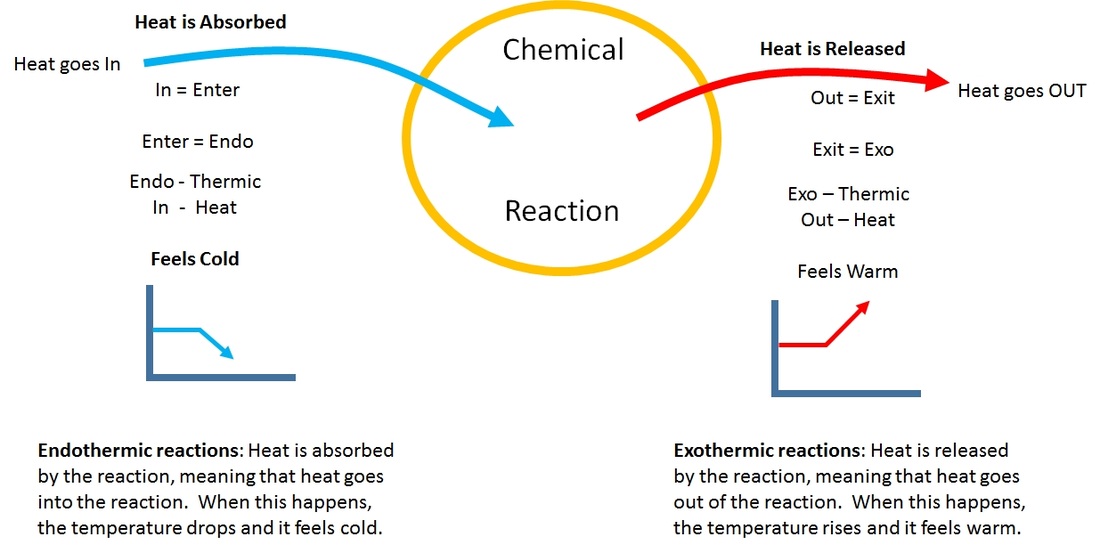
Endothermic Process In Solution . Heat energy is also needed to break the bonds in a solvent to insert. In both cases, step 1, separation of the. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Even if the solution is. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. An endothermic reaction is a chemical reaction that absorbs.
from vhmsscience.weebly.com
Heat energy is also needed to break the bonds in a solvent to insert. Even if the solution is. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). An endothermic reaction is a chemical reaction that absorbs. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. In both cases, step 1, separation of the. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at.
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE
Endothermic Process In Solution An endothermic reaction is a chemical reaction that absorbs. Even if the solution is. Heat energy is also needed to break the bonds in a solvent to insert. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. An endothermic reaction is a chemical reaction that absorbs. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). In both cases, step 1, separation of the. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases.
From www.clutchprep.com
Endothermic & Exothermic Reactions Chemistry Video Clutch Prep Endothermic Process In Solution In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. The enthalpy change of solution. Endothermic Process In Solution.
From en.ppt-online.org
Thermal Energy, Chemical Energy online presentation Endothermic Process In Solution To form a solution, energy is required to break the bonds between the particles within the solid or liquid. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Heat energy is also needed to break the bonds in a solvent to insert. Solvation can be an exothermic or. Endothermic Process In Solution.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Process In Solution Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Heat energy is also needed to break the bonds in a solvent to insert. Solvation can be an exothermic. Endothermic Process In Solution.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Endothermic Process In Solution Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Even if the solution is. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Some spontaneous processes. Endothermic Process In Solution.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Process In Solution In both cases, step 1, separation of the. Heat energy is also needed to break the bonds in a solvent to insert. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Some spontaneous processes. Endothermic Process In Solution.
From general.chemistrysteps.com
What is Enthalpy Chemistry Steps Endothermic Process In Solution To form a solution, energy is required to break the bonds between the particles within the solid or liquid. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. In both cases, step 1,. Endothermic Process In Solution.
From www.studypool.com
SOLUTION Endothermic and exothermic process Studypool Endothermic Process In Solution For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Heat energy is also needed to break the bonds in a solvent to insert. Even if the solution is. In both cases, step 1, separation of the. In the course of an endothermic process, the system gains heat from the surroundings and. Endothermic Process In Solution.
From www.youtube.com
Endothermic and exothermic reactions. Enthalpy YouTube Endothermic Process In Solution For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Heat energy is also needed to break the bonds in a solvent to insert. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. The enthalpy change of solution refers. Endothermic Process In Solution.
From keystagewiki.com
Endothermic Key Stage Wiki Endothermic Process In Solution Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). An endothermic reaction is a chemical reaction that absorbs. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. The enthalpy change of solution refers to the amount of heat that is released. Endothermic Process In Solution.
From www.slideserve.com
PPT Chapter 13 Properties of Solutions PowerPoint Presentation, free Endothermic Process In Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Solvation can be. Endothermic Process In Solution.
From www.pinterest.com
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Process In Solution Heat energy is also needed to break the bonds in a solvent to insert. In both cases, step 1, separation of the. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. Solvation can be an exothermic or. Endothermic Process In Solution.
From exolcytin.blob.core.windows.net
Endothermic Solution Process at Wilfred Hill blog Endothermic Process In Solution Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). In both cases, step 1, separation of the. The enthalpy change of solution refers to the amount of heat that is released or. Endothermic Process In Solution.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Process In Solution Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. In both cases, step 1, separation of the. Even if the solution is. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. The enthalpy change of solution. Endothermic Process In Solution.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Process In Solution For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Heat energy is also needed to break the bonds in a solvent to insert. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). An endothermic reaction is a chemical reaction that. Endothermic Process In Solution.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Process In Solution Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Even if the solution is.. Endothermic Process In Solution.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Process In Solution In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. An endothermic reaction is a chemical reaction that absorbs. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. The enthalpy change of solution refers to the amount of. Endothermic Process In Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic processes Studypool Endothermic Process In Solution To form a solution, energy is required to break the bonds between the particles within the solid or liquid. An endothermic reaction is a chemical reaction that absorbs. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Heat energy is also needed to break the bonds in a. Endothermic Process In Solution.
From courses.lumenlearning.com
11.1 The Dissolution Process General College Chemistry II Endothermic Process In Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. For example, the solution of sodium hydroxide is. Endothermic Process In Solution.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Process In Solution Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. Even if the solution is. Some spontaneous processes do not. Endothermic Process In Solution.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Process In Solution In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Even if the solution is. Some spontaneous processes do not involve the movement of the system to a lower energy. Endothermic Process In Solution.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Endothermic Process In Solution Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. An endothermic reaction is a chemical reaction that absorbs. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. In the course of an endothermic process, the system gains heat from the surroundings and. Endothermic Process In Solution.
From klasgnqsj.blob.core.windows.net
Endothermic Reaction Graph Example at Britt Fields blog Endothermic Process In Solution Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. Even if the solution is.. Endothermic Process In Solution.
From www.slideserve.com
PPT Energy in Chemical Changes PowerPoint Presentation, free download Endothermic Process In Solution Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. Heat energy is also needed to break the bonds in a solvent to insert. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. To form a solution, energy is required to break the bonds. Endothermic Process In Solution.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Process In Solution An endothermic reaction is a chemical reaction that absorbs. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). In both cases, step 1, separation of the. To form a solution, energy. Endothermic Process In Solution.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Process In Solution Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). To form a solution, energy is required to break the bonds between the particles within the solid or liquid. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. In both cases,. Endothermic Process In Solution.
From klajlbzfp.blob.core.windows.net
Endothermic Solution Examples at Jeffrey Pleasants blog Endothermic Process In Solution For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. To form a solution, energy is required to break the bonds between the particles within the solid or liquid. Heat. Endothermic Process In Solution.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Process In Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings. Endothermic Process In Solution.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Process In Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. In. Endothermic Process In Solution.
From www.slideserve.com
PPT TOPIC A States of Matter and Solutions PowerPoint Presentation Endothermic Process In Solution To form a solution, energy is required to break the bonds between the particles within the solid or liquid. An endothermic reaction is a chemical reaction that absorbs. Even if the solution is. In both cases, step 1, separation of the. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of. Endothermic Process In Solution.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Process In Solution The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. Heat energy is also needed to break the bonds in a solvent to insert. In both cases, step 1, separation of the. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent.. Endothermic Process In Solution.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Process In Solution In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process. Endothermic Process In Solution.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Endothermic Process In Solution Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). For example, the solution of sodium hydroxide is exothermic, and the solution of sodium chloride is somewhat endothermic. Solvation can be an exothermic or endothermic process depending on the nature of the solute and solvent. To form a solution, energy. Endothermic Process In Solution.
From www.studypool.com
SOLUTION Endothermic processes Studypool Endothermic Process In Solution Even if the solution is. In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases. In both cases, step 1, separation of the. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). An endothermic reaction is a. Endothermic Process In Solution.
From www.studypool.com
SOLUTION Exothermic and endothermic processes Studypool Endothermic Process In Solution Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. Some spontaneous processes do not involve the movement of the system to a lower energy state (e.g., an endothermic reaction). The enthalpy change of solution refers to the amount of heat that is released or absorbed during. Endothermic Process In Solution.
From mareeromana.blogspot.com
12+ Endothermic Enthalpy Diagram MareeRomana Endothermic Process In Solution Examples of endothermic reactions include photosynthesis, dissolving salt in water, and chemical cold packs. An endothermic reaction is a chemical reaction that absorbs. The enthalpy change of solution refers to the amount of heat that is released or absorbed during the dissolving process (at. In both cases, step 1, separation of the. In the course of an endothermic process, the. Endothermic Process In Solution.