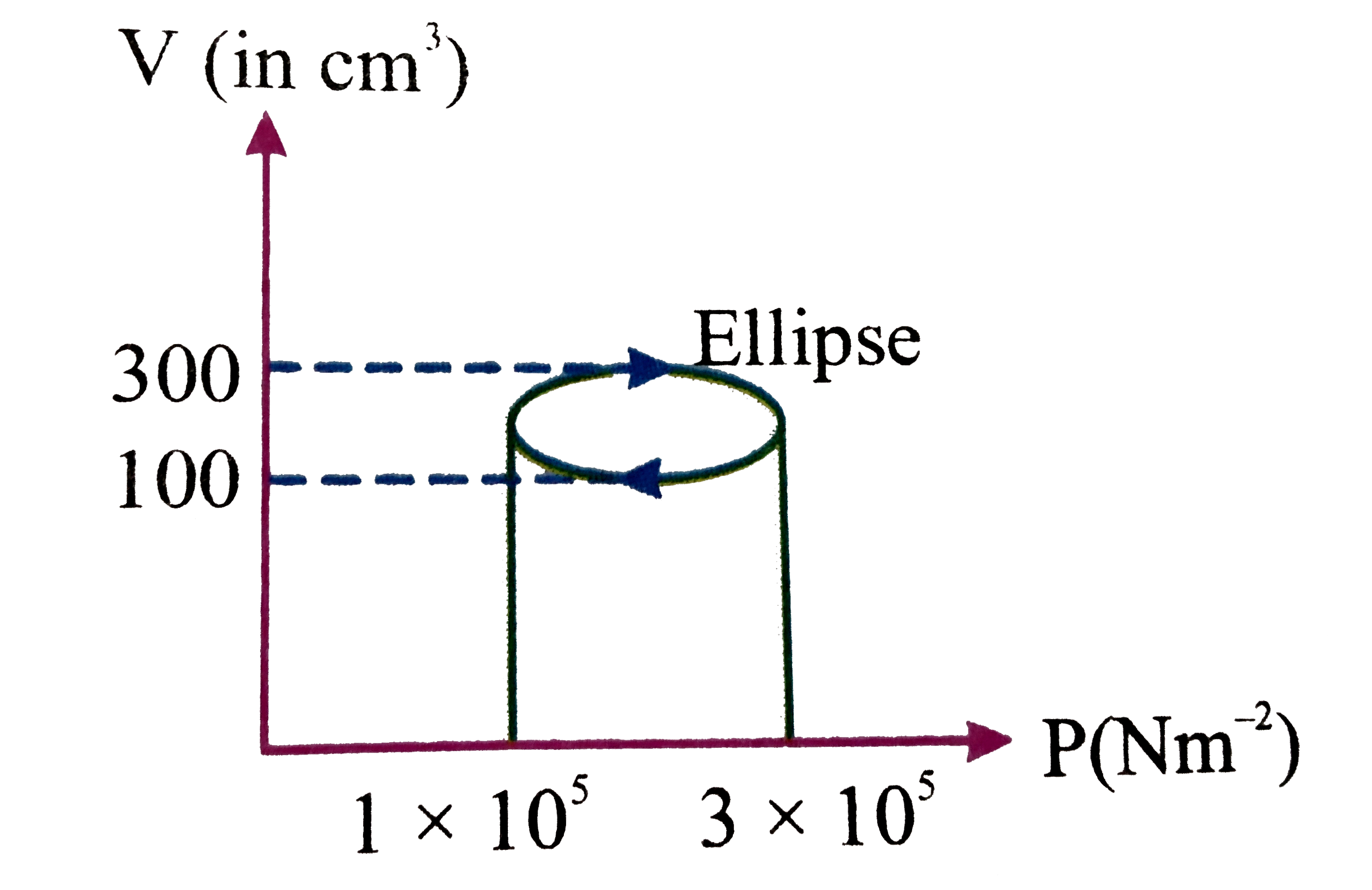
Heat Absorbed By The System In Going Through A Cyclic Process . Find the valur of x. This means that the heat absorbed by the system. Asked aug 3, 2021 in physics by vaiga ( 47.5k. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. Work done is equal to area of. Δq = δu + δw. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. Using the first law of thermodynamics, we get. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta w\] is the work done by the system. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. This makes the change in the internal energy of the system zero. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. Since internal energy depends only on the initial and final points and. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. In a cyclic process, the internal energy in a cycle remains constant.
from www.doubtnut.com
On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. In a cyclic process, the internal energy in a cycle remains constant. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. This makes the change in the internal energy of the system zero. Find the valur of x. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. Using the first law of thermodynamics, we get. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. Asked aug 3, 2021 in physics by vaiga ( 47.5k.
The heat absorbed by a system in going through the cyclic process show
Heat Absorbed By The System In Going Through A Cyclic Process Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. Since internal energy depends only on the initial and final points and. Asked aug 3, 2021 in physics by vaiga ( 47.5k. Find the valur of x. Work done is equal to area of. This means that the heat absorbed by the system. Δq = δu + δw. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. In a cyclic process, the internal energy in a cycle remains constant. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. Using the first law of thermodynamics, we get. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta w\] is the work done by the system. This makes the change in the internal energy of the system zero. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero.
From tardigrade.in
Calculate the heat absorbed by a system in going through the cyclic Heat Absorbed By The System In Going Through A Cyclic Process Since internal energy depends only on the initial and final points and. Asked aug 3, 2021 in physics by vaiga ( 47.5k. In a cyclic process, the internal energy in a cycle remains constant. This makes the change in the internal energy of the system zero. On the other hand, if a net amount of heat exits the system, it. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process Asked aug 3, 2021 in physics by vaiga ( 47.5k. In a cyclic process, the internal energy in a cycle remains constant. Since internal energy depends only on the initial and final points and. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. First law of thermodynamics,. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. This makes the change in the internal energy of the system zero. Δq = δu + δw. This means that the heat absorbed by the system. In a cyclic process, heat energy absorbed is equal to work. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.doubtnut.com
Calculate the heat absorbed by a system in going through the cycli Heat Absorbed By The System In Going Through A Cyclic Process This makes the change in the internal energy of the system zero. Since internal energy depends only on the initial and final points and. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. This means that the heat absorbed by the system. In a cyclic process, the. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process Find the valur of x. Since internal energy depends only on the initial and final points and. This means that the heat absorbed by the system. This makes the change in the internal energy of the system zero. Work done is equal to area of. In a cyclic process, the internal energy in a cycle remains constant. Δq = δu. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Net heat energy absorbed by the system in going through a cyclic Heat Absorbed By The System In Going Through A Cyclic Process Using the first law of thermodynamics, we get. Find the valur of x. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. This makes the change in the internal energy of the system zero. Work done is equal to area of. Since internal energy depends only on. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. In a cyclic process, the internal energy in a cycle remains constant. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta w\] is the work done by the system. Work done. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.doubtnut.com
The heat absorbed by the system in going through the cyclic process as Heat Absorbed By The System In Going Through A Cyclic Process On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. Since internal energy depends only on the initial and final points and. This makes the change. Heat Absorbed By The System In Going Through A Cyclic Process.
From kunduz.com
[ANSWERED] 9 Heat energy absorbed by a system in going through a cyclic Heat Absorbed By The System In Going Through A Cyclic Process This makes the change in the internal energy of the system zero. In a cyclic process, the internal energy in a cycle remains constant. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. Find the valur of x. Asked jun 7, 2019 in physics by jaishankarsahu (. Heat Absorbed By The System In Going Through A Cyclic Process.
From brainly.in
9. The heat energy absorbed in going through a circular cyclic process Heat Absorbed By The System In Going Through A Cyclic Process Find the valur of x. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta w\] is the work done by the system. This makes the change in the. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process Δq = δu + δw. Since internal energy depends only on the initial and final points and. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. This means that the heat absorbed by the system. Find the valur of x. This makes the change in the internal. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.youtube.com
The heat absorbed by the system in going through the cyclic process as Heat Absorbed By The System In Going Through A Cyclic Process In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.numerade.com
SOLVEDHeat energy absorbed by a system in going through a cyclic Heat Absorbed By The System In Going Through A Cyclic Process Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. Find the valur of x. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. This makes the change in the internal energy of the system zero. Work done is equal to area of. In a cyclic. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.youtube.com
Calculate the heat absorbed by a system in going through the cyclic Heat Absorbed By The System In Going Through A Cyclic Process This means that the heat absorbed by the system. Find the valur of x. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. Since internal energy depends only on the initial and final points and. In a cyclic process, the internal energy in a cycle remains constant.. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process This means that the heat absorbed by the system. In a cyclic process, the internal energy in a cycle remains constant. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. Δq = δu + δw. In the. Heat Absorbed By The System In Going Through A Cyclic Process.
From tardigrade.in
A system is taken through a cyclic process represented by a circle as Heat Absorbed By The System In Going Through A Cyclic Process Since internal energy depends only on the initial and final points and. Using the first law of thermodynamics, we get. Work done is equal to area of. In a cyclic process, the internal energy in a cycle remains constant. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. First law. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
The heat absorbed by the system in going through the cyclic process as Heat Absorbed By The System In Going Through A Cyclic Process On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. In a cyclic process, the internal energy in a cycle remains constant. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal. Heat Absorbed By The System In Going Through A Cyclic Process.
From askfilo.com
Heat energy absorbed by a system in going through a cyclic process shown Heat Absorbed By The System In Going Through A Cyclic Process This means that the heat absorbed by the system. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta w\] is the work done by the system. In a cyclic process, the internal energy in a cycle remains constant. Using the first law of thermodynamics, we get. Find the valur of. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. Work done is equal to area of. Asked jun 7, 2019 in physics by jaishankarsahu. Heat Absorbed By The System In Going Through A Cyclic Process.
From askfilo.com
Heat absorbed by a system in going through a cyclic process shown in figu.. Heat Absorbed By The System In Going Through A Cyclic Process Work done is equal to area of. Since internal energy depends only on the initial and final points and. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. Asked aug 3, 2021 in physics by vaiga ( 47.5k. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. Using the. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Calculate the heat absorbed by a system in going through the cyclic Heat Absorbed By The System In Going Through A Cyclic Process In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. Using the first law of thermodynamics, we get. This makes the change in the internal energy of the system zero. Find the valur of x. Since internal energy depends only on the initial and final points and. Work. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. In a cyclic process, the internal energy in a cycle remains constant. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. Asked aug 3, 2021 in physics by. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
The heat absorbed by the system in going through the cyclic process as Heat Absorbed By The System In Going Through A Cyclic Process Work done is equal to area of. This means that the heat absorbed by the system. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta w\] is the work done by the system. Δq = δu + δw. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. Find the. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process Work done is equal to area of. First law of thermodynamics, where \[\delta q\] is the heat absorbed, \[\delta u\] is the internal energy and \[\delta w\] is the work done by the system. In a cyclic process, the internal energy in a cycle remains constant. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. In a cyclic process,. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process Asked aug 3, 2021 in physics by vaiga ( 47.5k. Using the first law of thermodynamics, we get. In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. Find the valur of x. Work done is equal to area of. In the reported figure, two bodies a and. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.youtube.com
calculate the heat absorbed by a system in going through the cuyclic Heat Absorbed By The System In Going Through A Cyclic Process In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. Work done is equal to area of. Since internal energy depends only on the initial and final points and.. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.doubtnut.com
Heat energy absorbed by a system in going through a cyclic process sho Heat Absorbed By The System In Going Through A Cyclic Process Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. Asked aug 3, 2021 in. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
2. Heat absorbed by the system is going through a cyclic process as Heat Absorbed By The System In Going Through A Cyclic Process On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. Work done is equal to area of. Find the valur of x. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. Using the first law of thermodynamics, we. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.doubtnut.com
Calculate the heat absorbed by a system in going through the cycli Heat Absorbed By The System In Going Through A Cyclic Process This means that the heat absorbed by the system. Find the valur of x. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. In a cyclic process, the internal energy in a cycle remains constant. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. In the. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.doubtnut.com
The heat absorbed by a system in going through the cyclic process show Heat Absorbed By The System In Going Through A Cyclic Process In a cyclic process, heat energy absorbed is equal to work done as change in internal energy of the system is zero. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. Find the valur of x. This means that the heat absorbed by the system. Δq = δu + δw. Since internal energy depends only on the initial and. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.doubtnut.com
Heat energy absorbed by a system in going through a cyclic process sho Heat Absorbed By The System In Going Through A Cyclic Process The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. In a cyclic process, the internal energy in a cycle remains constant. On the other hand, if a net amount of heat exits the system, it must have entered in the form of work. Find the valur of x. This means. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.doubtnut.com
Calculate the heat abosrbed by a system in going through the cyclic pr Heat Absorbed By The System In Going Through A Cyclic Process The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. In a cyclic process, the internal energy in a cycle remains constant. Using the first law of thermodynamics, we get. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. This makes the change in the internal energy of the system. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process Δq = δu + δw. This means that the heat absorbed by the system. In a cyclic process, the internal energy in a cycle remains constant. The heat absorbed by a system in going through the cyclic process shown shown in fig is `xxx 5pij`. Since internal energy depends only on the initial and final points and. Asked jun 7,. Heat Absorbed By The System In Going Through A Cyclic Process.
From www.toppr.com
Heat energy absorbed by a system in going through a cyclic process Heat Absorbed By The System In Going Through A Cyclic Process This makes the change in the internal energy of the system zero. Asked jun 7, 2019 in physics by jaishankarsahu ( 86.0k. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. This means that the heat absorbed by the system. First law of thermodynamics, where \[\delta. Heat Absorbed By The System In Going Through A Cyclic Process.
From askfilo.com
Heat energy absorbed by a system is going through a cyclic process is sho.. Heat Absorbed By The System In Going Through A Cyclic Process Since internal energy depends only on the initial and final points and. In the reported figure, two bodies a and b of masses 200 g and 800 g are attached with the system of springs. Asked aug 3, 2021 in physics by vaiga ( 47.5k. Δq = δu + δw. This means that the heat absorbed by the system. This. Heat Absorbed By The System In Going Through A Cyclic Process.