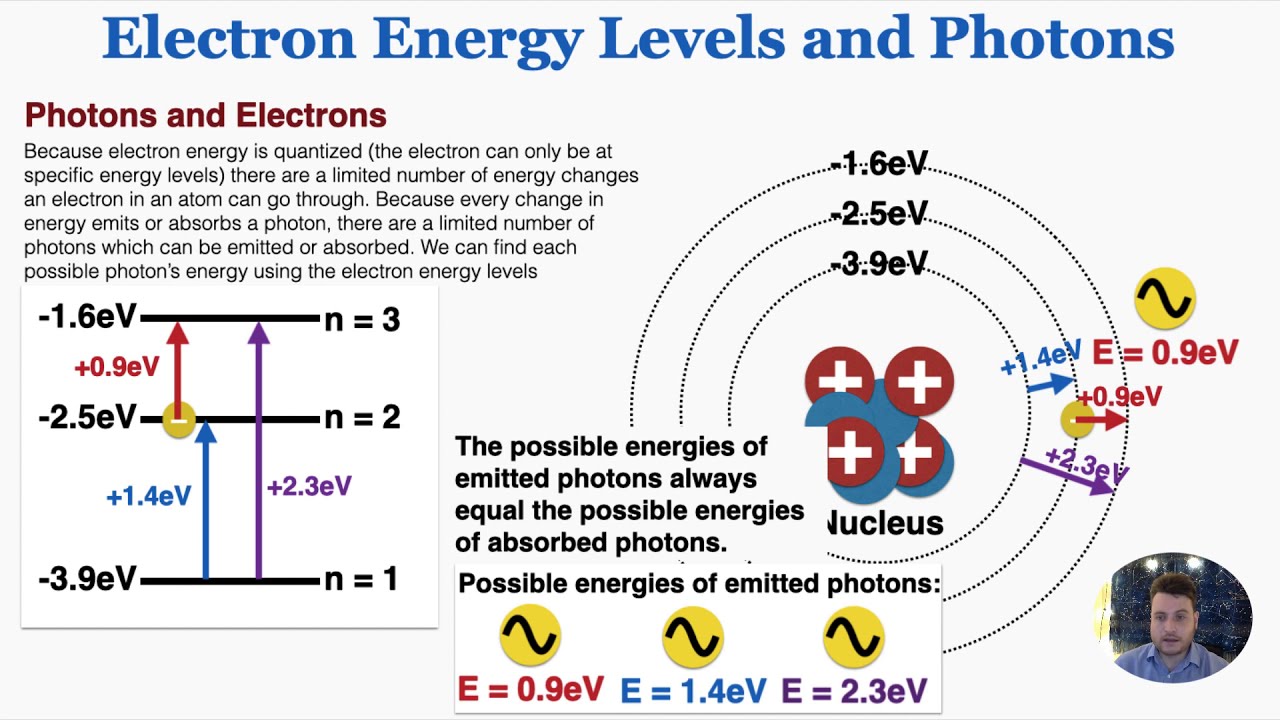
What Happens When A Photon Is Absorbed By An Atom . The atomic system supports multiple energy levels; In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. Consider white light incident on a material. The process results in ionization by subsequent ejection of the electron from the atom. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. When the photon is absorbed, the system gains energy. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy.
from www.youtube.com
When the photon is absorbed, the system gains energy. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Consider white light incident on a material. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. The process results in ionization by subsequent ejection of the electron from the atom. The atomic system supports multiple energy levels;
Electron Energy Levels and Photons IB Physics YouTube
What Happens When A Photon Is Absorbed By An Atom In photoelectric absorption, a photon disappears being absorbed by an atomic electron. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. The atomic system supports multiple energy levels; The process results in ionization by subsequent ejection of the electron from the atom. When an electron jumps from a. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. Consider white light incident on a material. When the photon is absorbed, the system gains energy. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. In photoelectric absorption, a photon disappears being absorbed by an atomic electron.
From www.britannica.com
Compton effect Definition, Formula, & Facts Britannica What Happens When A Photon Is Absorbed By An Atom In photoelectric absorption, a photon disappears being absorbed by an atomic electron. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. When a photon. What Happens When A Photon Is Absorbed By An Atom.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube What Happens When A Photon Is Absorbed By An Atom Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. Consider white light incident on a material. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a photon is absorbed, it transfers transverse wave energy. What Happens When A Photon Is Absorbed By An Atom.
From physicsinreallife.blogspot.com
Everyday Physics Energy Per Photon What Happens When A Photon Is Absorbed By An Atom In photoelectric absorption, a photon disappears being absorbed by an atomic electron. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When an electron jumps from a. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result. What Happens When A Photon Is Absorbed By An Atom.
From circuitdiagramgyte.z22.web.core.windows.net
Draw Energy Level Diagram Of Hydrogen Atom What Happens When A Photon Is Absorbed By An Atom Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. When an electron jumps from a. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. When the photon is absorbed, the system gains energy. In photoelectric absorption,. What Happens When A Photon Is Absorbed By An Atom.
From www.numerade.com
SOLVED In Thomson's plum pudding model, what happens when the photons What Happens When A Photon Is Absorbed By An Atom In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons. What Happens When A Photon Is Absorbed By An Atom.
From www.astronomynotes.com
Radiation What Happens When A Photon Is Absorbed By An Atom In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When an electron jumps from a. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. The process results. What Happens When A Photon Is Absorbed By An Atom.
From www.numerade.com
SOLVEDThe interaction of light with matter deals directly with the What Happens When A Photon Is Absorbed By An Atom Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed. What Happens When A Photon Is Absorbed By An Atom.
From www.nagwa.com
Question Video Finding the Required Wavelength of an Absorbed Photon What Happens When A Photon Is Absorbed By An Atom Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When the photon is absorbed, the system gains energy. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. Photons of particular wavelength can be absorbed by an. What Happens When A Photon Is Absorbed By An Atom.
From www.youtube.com
Bohr Calculation Example 1 find ΔE and the wavelength of the photon What Happens When A Photon Is Absorbed By An Atom Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a photon is absorbed, it transfers transverse wave energy to longitudinal. What Happens When A Photon Is Absorbed By An Atom.
From www.vrogue.co
Calculate The Energy Frequency Wavelength Of An Elect vrogue.co What Happens When A Photon Is Absorbed By An Atom When the photon is absorbed, the system gains energy. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. The process results in ionization by subsequent ejection. What Happens When A Photon Is Absorbed By An Atom.
From courses.lumenlearning.com
The LightDependent Reactions of Photosynthesis Biology I What Happens When A Photon Is Absorbed By An Atom In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. The process results in ionization by subsequent ejection of the electron from the atom. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of. What Happens When A Photon Is Absorbed By An Atom.
From www.youtube.com
ABC Zoom Electrons and photons absorption and transmission of light What Happens When A Photon Is Absorbed By An Atom In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When the photon is absorbed, the system gains energy. The atomic system supports multiple energy levels; When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the. What Happens When A Photon Is Absorbed By An Atom.
From www.youtube.com
What is the wavelength of light emitted when the electron in a hydrogen What Happens When A Photon Is Absorbed By An Atom In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. The atomic system supports multiple energy levels; Consider white light incident on. What Happens When A Photon Is Absorbed By An Atom.
From buoitutrung.com
Are Atoms Made Of Photons? Exploring The Elemental Mystery Vườn Bưởi What Happens When A Photon Is Absorbed By An Atom When the photon is absorbed, the system gains energy. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a photon is absorbed, it transfers transverse. What Happens When A Photon Is Absorbed By An Atom.
From www.nagwa.com
Question Video Finding Which Diagram More Correctly Shows Changes in What Happens When A Photon Is Absorbed By An Atom There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. Consider white light incident on a material. When the. What Happens When A Photon Is Absorbed By An Atom.
From www.nachi.org
Atomic Valence Shells Energy Emittance Inspection Gallery InterNACHI® What Happens When A Photon Is Absorbed By An Atom When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a. What Happens When A Photon Is Absorbed By An Atom.
From www.slideserve.com
PPT Matter and Particles of Light Quantum Theory PowerPoint What Happens When A Photon Is Absorbed By An Atom When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. Consider white light incident on a material. In photoelectric absorption, a photon disappears being absorbed. What Happens When A Photon Is Absorbed By An Atom.
From en.rattibha.com
⚛️PHYS001 Basics of atomic physics and fission. In this short thread What Happens When A Photon Is Absorbed By An Atom The process results in ionization by subsequent ejection of the electron from the atom. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. When an electron jumps from a. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. When a photon hits an electron,both moving in the same direction,. What Happens When A Photon Is Absorbed By An Atom.
From energywavetheory.com
Photon Creation and Absorption EWT What Happens When A Photon Is Absorbed By An Atom In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. When the photon is absorbed,. What Happens When A Photon Is Absorbed By An Atom.
From general.chemistrysteps.com
Rydberg Formula Chemistry Steps What Happens When A Photon Is Absorbed By An Atom When an electron jumps from a. When the photon is absorbed, the system gains energy. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. In photoelectric. What Happens When A Photon Is Absorbed By An Atom.
From webmis.highland.cc.il.us
Atomic Spectra and Models of the Atom What Happens When A Photon Is Absorbed By An Atom In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. Photons of particular wavelength can be absorbed by an atom by causing an electron. What Happens When A Photon Is Absorbed By An Atom.
From giozzittk.blob.core.windows.net
Distinguish Between Conducted Emission And Radiated Emission at Latasha What Happens When A Photon Is Absorbed By An Atom Consider white light incident on a material. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. The atomic system supports multiple energy levels; When a photon is absorbed, it transfers. What Happens When A Photon Is Absorbed By An Atom.
From climate.nasa.gov
Seeing photosynthesis from space NASA scientists use satellites to What Happens When A Photon Is Absorbed By An Atom Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. When an electron jumps from a. The atomic system supports multiple energy levels; When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. There are several options. What Happens When A Photon Is Absorbed By An Atom.
From www.numerade.com
SOLVED Consider these two cases Case 1 An electron jumps from energy What Happens When A Photon Is Absorbed By An Atom When an electron jumps from a. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a. What Happens When A Photon Is Absorbed By An Atom.
From chem.libretexts.org
7.1 Vocabulary Chemistry LibreTexts What Happens When A Photon Is Absorbed By An Atom Consider white light incident on a material. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. There are several options that could happen. What Happens When A Photon Is Absorbed By An Atom.
From general.chemistrysteps.com
Calculating The Energy of a Photon Chemistry Steps What Happens When A Photon Is Absorbed By An Atom When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. The process results in ionization by subsequent ejection of the electron from the atom. The atomic system supports multiple energy levels; In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy. What Happens When A Photon Is Absorbed By An Atom.
From general.chemistrysteps.com
Bohr Model of the Hydrogen Atom Chemistry Steps What Happens When A Photon Is Absorbed By An Atom The atomic system supports multiple energy levels; In photoelectric absorption, a photon disappears being absorbed by an atomic electron. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light. What Happens When A Photon Is Absorbed By An Atom.
From study.com
Electron Transition Definition, Chart & Examples Lesson What Happens When A Photon Is Absorbed By An Atom There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. When an electron jumps from a. The atomic system supports multiple energy levels; Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels.. What Happens When A Photon Is Absorbed By An Atom.
From www.sciencenews.org
Colliding photons made matter. But are the photons ‘real’? Science News What Happens When A Photon Is Absorbed By An Atom The process results in ionization by subsequent ejection of the electron from the atom. When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy —. What Happens When A Photon Is Absorbed By An Atom.
From www.chegg.com
Solved What do you think would happen to the electron when a What Happens When A Photon Is Absorbed By An Atom In physics, absorption of electromagnetic radiation is how matter (typically electrons bound in atoms) takes up a photon's energy — and so transforms electromagnetic energy into. When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. When the photon is absorbed, the system gains energy. The atomic system supports multiple. What Happens When A Photon Is Absorbed By An Atom.
From phys.org
Mapping the interaction of a single atom with a single photon may What Happens When A Photon Is Absorbed By An Atom When a photon is absorbed, it transfers transverse wave energy to longitudinal energy as a result of the electron’s spin. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. Photons of particular. What Happens When A Photon Is Absorbed By An Atom.
From circuitestrilhatc6.z13.web.core.windows.net
Diagram Of Hydrogen Atom What Happens When A Photon Is Absorbed By An Atom Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. In photoelectric absorption, a photon disappears being absorbed by an atomic electron. There are several options that could happen next, either. What Happens When A Photon Is Absorbed By An Atom.
From energywavetheory.com
Photon Creation and Absorption EWT What Happens When A Photon Is Absorbed By An Atom When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. The atomic system supports multiple energy levels; There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. In physics,. What Happens When A Photon Is Absorbed By An Atom.
From www.khadley.com
hydrogen spectrum What Happens When A Photon Is Absorbed By An Atom The process results in ionization by subsequent ejection of the electron from the atom. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Photons of particular wavelength can be absorbed by an atom by causing an electron to jump from. There are several options that could happen. What Happens When A Photon Is Absorbed By An Atom.
From exouyokim.blob.core.windows.net
Why Do Leaves Not Absorb Green Light at Wanda Perdue blog What Happens When A Photon Is Absorbed By An Atom When a photon hits an electron,both moving in the same direction, the photon will be partially absorbed and the electron emits another photon with lower energy. There are several options that could happen next, either the electron returns to the ground state emitting the photon of light or the energy is. When a photon is absorbed, it transfers transverse wave. What Happens When A Photon Is Absorbed By An Atom.