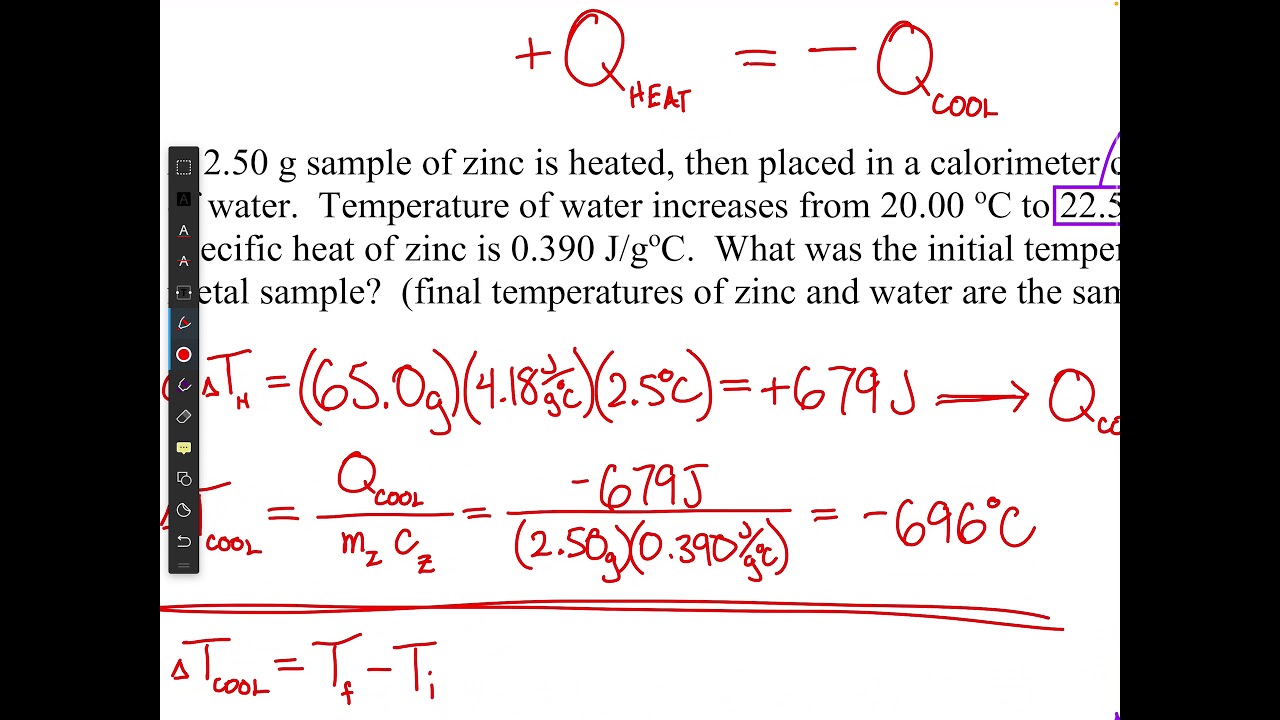Lab Calorimetry And Specific Heat Conclusion . • use scientific reasoning skills (such as observing,. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. Calorimetry is the science of measuring heat flow. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in temperature (δt). • perform calculations involving change in temperature, specific heat capacity, and heat. For example, if you drop a coin into a cup with hot water, the temperature of the coin will go up until it is at the same temperature as the boiling water. The specific heat of a. In this experiment, students will find the specific heat of three “unknown” samples of metal. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. To do this, students will use their knowledge of. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. The specific heat test described in this article required many modifications and repetitions to display conclusive results.
from www.youtube.com
The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. Calorimetry is the science of measuring heat flow. The specific heat test described in this article required many modifications and repetitions to display conclusive results. • use scientific reasoning skills (such as observing,. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. To do this, students will use their knowledge of. In this experiment, students will find the specific heat of three “unknown” samples of metal. • perform calculations involving change in temperature, specific heat capacity, and heat.
G10 Chemistry Calorimetry and Specific Heat Capacity Worked Examples
Lab Calorimetry And Specific Heat Conclusion The specific heat of a. To do this, students will use their knowledge of. • perform calculations involving change in temperature, specific heat capacity, and heat. The specific heat test described in this article required many modifications and repetitions to display conclusive results. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. In this experiment, students will find the specific heat of three “unknown” samples of metal. Calorimetry is the science of measuring heat flow. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in temperature (δt). If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. The specific heat of a. For example, if you drop a coin into a cup with hot water, the temperature of the coin will go up until it is at the same temperature as the boiling water. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. • use scientific reasoning skills (such as observing,. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Lab Calorimetry And Specific Heat Conclusion • use scientific reasoning skills (such as observing,. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. In this experiment, students will find the specific heat of three “unknown” samples of metal. Heat is defined as thermal energy flowing from an object at a higher temperature to one at. Lab Calorimetry And Specific Heat Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Lab Calorimetry And Specific Heat Conclusion Calorimetry is the science of measuring heat flow. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. The specific heat of a. The major results for the three quantitative measurements include the average heat energy (. Lab Calorimetry And Specific Heat Conclusion.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab calorimetry and specific heat student Lab Calorimetry And Specific Heat Conclusion The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. To do this, students will use their knowledge of. The specific heat test described in this article required many modifications and repetitions to display conclusive results. If a metal has a low specific heat, then the metal could make an excellent material. Lab Calorimetry And Specific Heat Conclusion.
From studylib.net
Calorimetry Lab Specific Heat Capacity Lab Calorimetry And Specific Heat Conclusion The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. In this experiment, students will find the specific heat of three “unknown” samples of metal. The amount. Lab Calorimetry And Specific Heat Conclusion.
From webapi.bu.edu
💣 Specific heat of a metal lab conclusion. Specific Heat of Metal Lab Lab Calorimetry And Specific Heat Conclusion To do this, students will use their knowledge of. The specific heat test described in this article required many modifications and repetitions to display conclusive results. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. The major results for the three quantitative measurements include the average heat energy (. Lab Calorimetry And Specific Heat Conclusion.
From www.youtube.com
Specific Heat of Metal Sample Calorimetry Lab Problem solved YouTube Lab Calorimetry And Specific Heat Conclusion The specific heat test described in this article required many modifications and repetitions to display conclusive results. To do this, students will use their knowledge of. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. • perform calculations involving change in temperature, specific heat capacity, and. Lab Calorimetry And Specific Heat Conclusion.
From kaffee.50webs.com
Lab Calorimetry Lab Calorimetry And Specific Heat Conclusion The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The specific heat test described in this article required many modifications and repetitions to display conclusive results. The specific heat of a. • use scientific reasoning skills (such as observing,. If a metal has a low specific heat, then the metal could. Lab Calorimetry And Specific Heat Conclusion.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Lab Calorimetry And Specific Heat Conclusion The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. Calorimetry is the science of measuring heat flow. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. • use scientific reasoning skills (such as observing,. To do this, students will use. Lab Calorimetry And Specific Heat Conclusion.
From www.scribd.com
Lab 07Specific Heat & Calorimetry PDF PDF Heat Temperature Lab Calorimetry And Specific Heat Conclusion The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. • use scientific reasoning skills (such as observing,. The specific heat test described in this article required many modifications and repetitions to display conclusive results. In this experiment, students will find the specific heat of three “unknown” samples of metal. The results. Lab Calorimetry And Specific Heat Conclusion.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Lab Calorimetry And Specific Heat Conclusion To do this, students will use their knowledge of. For example, if you drop a coin into a cup with hot water, the temperature of the coin will go up until it is at the same temperature as the boiling water. • use scientific reasoning skills (such as observing,. Heat is defined as thermal energy flowing from an object at. Lab Calorimetry And Specific Heat Conclusion.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Lab Calorimetry And Specific Heat Conclusion The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. Calorimetry is the science of measuring heat flow. In this experiment, students will find the specific heat of three “unknown” samples of metal. For example, if you drop a coin into a cup with hot water, the temperature of the. Lab Calorimetry And Specific Heat Conclusion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Lab Calorimetry And Specific Heat Conclusion • use scientific reasoning skills (such as observing,. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. Calorimetry is the science of measuring heat flow. The specific heat of a. For example, if you drop a coin into a cup with hot water, the temperature of. Lab Calorimetry And Specific Heat Conclusion.
From www.studypool.com
SOLUTION Lab calorimetry and specific heat test 2023 Studypool Lab Calorimetry And Specific Heat Conclusion • perform calculations involving change in temperature, specific heat capacity, and heat. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. • use scientific reasoning skills (such as observing,. In this. Lab Calorimetry And Specific Heat Conclusion.
From brainly.com
please i need it Lab Calorimetry and Specific Heat Lab Calorimetry And Specific Heat Conclusion If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. The specific heat of a. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware. Lab Calorimetry And Specific Heat Conclusion.
From studylib.net
Lab 11b Specific Heat & Heat Capacity Lab Calorimetry And Specific Heat Conclusion In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. To do this, students will use their knowledge of. The amount of heat absorbed or released (q). Lab Calorimetry And Specific Heat Conclusion.
From www.studypool.com
SOLUTION Lab report calorimetry and specific heat Studypool Lab Calorimetry And Specific Heat Conclusion The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in temperature (δt). The specific heat of a. For example, if you drop a coin into a cup with hot water, the temperature of the coin will go up until it is at the same temperature as. Lab Calorimetry And Specific Heat Conclusion.
From courses.lumenlearning.com
Calorimetry Introductory Chemistry Lecture & Lab Lab Calorimetry And Specific Heat Conclusion The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. Calorimetry is the science of measuring heat flow. • use scientific reasoning skills (such as observing,. The specific heat test described in this article required many modifications and repetitions to display conclusive results. If a metal has a low specific. Lab Calorimetry And Specific Heat Conclusion.
From www.numerade.com
SOLVED Chem 1215L, Report Sheet Lab Calorimetry Specific Heat of a Lab Calorimetry And Specific Heat Conclusion The specific heat of a. Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. • perform calculations involving change in temperature, specific heat capacity, and heat. Calorimetry is the science of measuring heat flow. In this experiment, students will find the specific heat of three “unknown” samples of metal.. Lab Calorimetry And Specific Heat Conclusion.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Lab Calorimetry And Specific Heat Conclusion The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Calorimetry and Specific Heat Lab Calorimetry and Specific Heat Lab Calorimetry And Specific Heat Conclusion The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The specific heat test described in this article required many modifications and repetitions to display conclusive results. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Calorimetry and Specific Heat Lab Report Anna Mitchell Chemistry 11 Lab Calorimetry And Specific Heat Conclusion Calorimetry is the science of measuring heat flow. The specific heat of a. In this experiment, students will find the specific heat of three “unknown” samples of metal. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. • perform calculations involving change in temperature, specific heat capacity, and heat.. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Lab Calorimetry and Specific Heat Chemistry A 29 December 2023 Lab Lab Calorimetry And Specific Heat Conclusion To do this, students will use their knowledge of. In this experiment, students will find the specific heat of three “unknown” samples of metal. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of. Lab Calorimetry And Specific Heat Conclusion.
From studylib.net
Experiment 15 Specific Heat of a Metal Lab Calorimetry And Specific Heat Conclusion To do this, students will use their knowledge of. The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The specific heat test described in this article required many modifications and repetitions to display conclusive results. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead. Lab Calorimetry And Specific Heat Conclusion.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab calorimetry and specific heat student Lab Calorimetry And Specific Heat Conclusion Calorimetry is the science of measuring heat flow. If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Lab Calorimetry and Specific Heat SCIENCE LAB REPORT Please go to Lab Calorimetry And Specific Heat Conclusion The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. The specific heat of a. • perform calculations involving change in temperature, specific heat capacity, and heat. Calorimetry is the science of measuring heat flow. In this experiment, students will find the specific heat of three “unknown” samples of metal. The results. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Calorimetry and Specific Heat Lab Question Using a calorimeter, how Lab Calorimetry And Specific Heat Conclusion • use scientific reasoning skills (such as observing,. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. Calorimetry is the science of measuring heat flow. The specific heat of a. The amount of heat absorbed or released (q) by the object depends on its mass (m),. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
P calorimetry 25 lab report StuDocu Lab Calorimetry And Specific Heat Conclusion • use scientific reasoning skills (such as observing,. To do this, students will use their knowledge of. In this experiment, students will find the specific heat of three “unknown” samples of metal. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. For example, if you drop. Lab Calorimetry And Specific Heat Conclusion.
From janiyahabbgates.blogspot.com
Calorimetry Specific Heat Capacity of Metals Lab Report JaniyahabbGates Lab Calorimetry And Specific Heat Conclusion In this experiment, students will find the specific heat of three “unknown” samples of metal. • use scientific reasoning skills (such as observing,. • perform calculations involving change in temperature, specific heat capacity, and heat. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in temperature. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Lab 1 calorimetry of reactions and heat transfer chem 1302 Studocu Lab Calorimetry And Specific Heat Conclusion For example, if you drop a coin into a cup with hot water, the temperature of the coin will go up until it is at the same temperature as the boiling water. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. The amount of heat absorbed. Lab Calorimetry And Specific Heat Conclusion.
From brainly.com
Does anyone have the table for the Calorimetry and Specific Heat lab Lab Calorimetry And Specific Heat Conclusion Heat is defined as thermal energy flowing from an object at a higher temperature to one at a lower temperature. In this experiment, students will find the specific heat of three “unknown” samples of metal. In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. • perform. Lab Calorimetry And Specific Heat Conclusion.
From www.studypool.com
SOLUTION Chemistry b unit 2 lab calorimetry and specific heat student Lab Calorimetry And Specific Heat Conclusion Calorimetry is the science of measuring heat flow. For example, if you drop a coin into a cup with hot water, the temperature of the coin will go up until it is at the same temperature as the boiling water. The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups.. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Lab Calorimetry And Specific Heat Conclusion If a metal has a low specific heat, then the metal could make an excellent material for cookware because it takes less heat to raise the temperature of the metal to cook food. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat (c s), and the change in temperature (δt). Heat. Lab Calorimetry And Specific Heat Conclusion.
From www.youtube.com
Energy 5 Calorimetry/Specific Heat Lab YouTube Lab Calorimetry And Specific Heat Conclusion The results obtained were relatively accurate even taking into consideration the modification of using a calorimeter made of styrofoam cups. The specific heat of a. The specific heat test described in this article required many modifications and repetitions to display conclusive results. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific heat. Lab Calorimetry And Specific Heat Conclusion.
From www.studocu.com
Copy of Lab 5 Calorimetry and Specific Heat Lab Calorimetry and Lab Calorimetry And Specific Heat Conclusion The major results for the three quantitative measurements include the average heat energy ( t)δ within the substances. To do this, students will use their knowledge of. Calorimetry is the science of measuring heat flow. • use scientific reasoning skills (such as observing,. The amount of heat absorbed or released (q) by the object depends on its mass (m), specific. Lab Calorimetry And Specific Heat Conclusion.
From www.youtube.com
G10 Chemistry Calorimetry and Specific Heat Capacity Worked Examples Lab Calorimetry And Specific Heat Conclusion In conclusion, this lab focused on identifying the specific heat of aluminum, copper, iron, and lead to choose the most fitting cookware material. In this experiment, students will find the specific heat of three “unknown” samples of metal. Calorimetry is the science of measuring heat flow. • perform calculations involving change in temperature, specific heat capacity, and heat. • use. Lab Calorimetry And Specific Heat Conclusion.