What Is The Point Of The Electrode . Standard electrode potential is a measurement of the potential for equilibrium. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrodes are vital components of electrochemical cells. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. They’re the positively and negatively charged halves of the system. Each cell has two electrodes.
from chem.libretexts.org
An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. Electrodes are vital components of electrochemical cells. They’re the positively and negatively charged halves of the system. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Each cell has two electrodes.
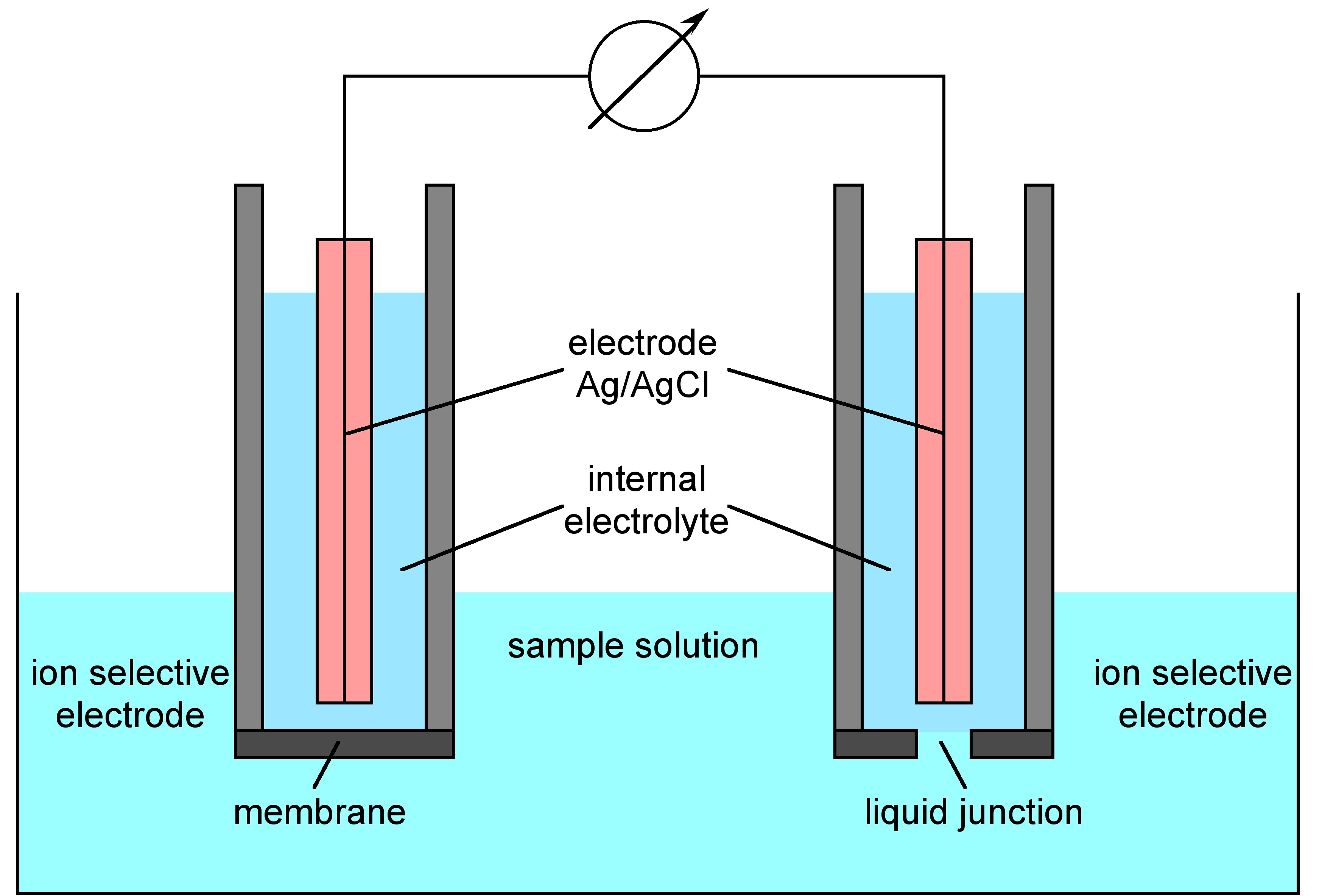
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts
What Is The Point Of The Electrode Each cell has two electrodes. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. They’re the positively and negatively charged halves of the system. Electrodes are vital components of electrochemical cells. Each cell has two electrodes. Standard electrode potential is a measurement of the potential for equilibrium. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium;
From litfl.com
ECG Lead positioning • LITFL • ECG Library Basics What Is The Point Of The Electrode Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrodes are vital components of electrochemical cells. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Each cell. What Is The Point Of The Electrode.
From www.britannica.com
Electricity Deriving, Electric Field, Potential Britannica What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. Electrodes are vital components of electrochemical cells. Each cell has two electrodes. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. They’re the positively. What Is The Point Of The Electrode.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Standard electrode potential is a measurement of the potential for equilibrium. Each cell has two electrodes. They’re the positively and negatively charged halves of the system.. What Is The Point Of The Electrode.
From www.researchgate.net
Fourpoint electrode configuration with current and potential [31 What Is The Point Of The Electrode Electrodes are vital components of electrochemical cells. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; They’re the positively and negatively charged halves of the system. Standard electrode potential is a measurement of the potential for equilibrium. Each cell has two electrodes. There is a potential difference between the electrode and the. What Is The Point Of The Electrode.
From alevelchemistry.co.uk
Electrodes Facts, Summary & Definition Chemistry Revision What Is The Point Of The Electrode Each cell has two electrodes. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are vital components of electrochemical cells. They’re the positively and negatively charged halves of the system. Standard electrode potential is a measurement of the potential for equilibrium. An electrode reaction refers to the net oxidation or reduction. What Is The Point Of The Electrode.
From www.researchgate.net
Electrode structure. (a) Schematic of the needle to plane electrodes What Is The Point Of The Electrode Each cell has two electrodes. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Standard electrode potential is a measurement of the potential for equilibrium. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrode, electric conductor, usually metal, used as either of the. What Is The Point Of The Electrode.
From mungfali.com
Electrode Potential Series What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. Each cell has two electrodes. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are vital components of electrochemical cells. They’re the positively. What Is The Point Of The Electrode.
From www.researchgate.net
Electrode design details (a) schematic of electrode designs and (b What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically. What Is The Point Of The Electrode.
From www.youtube.com
6 Different Types of Electrodes & their Reactions in Electrochemistry What Is The Point Of The Electrode Each cell has two electrodes. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are vital components of electrochemical cells. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. An electrode reaction refers to the net oxidation or reduction process that takes place. What Is The Point Of The Electrode.
From www.researchgate.net
Schematic diagrams of electrode arrays and their sensitivity patterns What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. They’re the positively and negatively charged halves of the system. Standard electrode potential is a measurement of the potential for equilibrium. Each cell has two electrodes. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode.. What Is The Point Of The Electrode.
From www.researchgate.net
22 Schematic of (a) twoelectrode and (b) threeelectrode... Download What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode reaction refers to the net oxidation or reduction process that takes place at. What Is The Point Of The Electrode.
From www.researchgate.net
2 A conventional setup with three electrodes of an electrochemical cell What Is The Point Of The Electrode An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Each cell has two electrodes. Standard electrode potential is a measurement of the potential for equilibrium. Electrodes are vital components of electrochemical cells. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrode, electric conductor,. What Is The Point Of The Electrode.
From www.snexplores.org
Explainer What is an electrode? What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Each cell has two electrodes. They’re the positively and negatively charged halves of the system. Electrodes are vital components of electrochemical cells. Standard electrode potential is a measurement of the potential for equilibrium. An electrode reaction refers to the net oxidation or reduction. What Is The Point Of The Electrode.
From theweldings.com
Welding Electrodes Types, functions and Definition Weld World What Is The Point Of The Electrode They’re the positively and negatively charged halves of the system. Each cell has two electrodes. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrodes are vital components of electrochemical cells. Standard electrode potential is. What Is The Point Of The Electrode.
From www.researchgate.net
Solid contact electrode design and crosssection of the electrode area What Is The Point Of The Electrode An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. They’re the positively and negatively charged halves of the system. Electrode, electric conductor, usually metal,. What Is The Point Of The Electrode.
From www.researchgate.net
Intramuscular electrodes. a Top view of the electrodes showing a distal What Is The Point Of The Electrode An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Each cell has two electrodes. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. They’re the positively and negatively charged halves of the system. Electrode, electric conductor, usually metal, used as either of the two. What Is The Point Of The Electrode.
From inmindout.com
Neurofeedback How and Where to Place Electrodes InMindOut Emotional What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrodes are vital components of electrochemical cells. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Standard electrode potential is a measurement of the potential for equilibrium. They’re the positively and negatively charged halves of. What Is The Point Of The Electrode.
From electrical-engineering-portal.com
Learn earth ground resistance principles, testing methods and applications What Is The Point Of The Electrode Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Standard electrode potential is a measurement of the potential for equilibrium. Electrodes are vital components of electrochemical cells. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. An electrode reaction refers to the net oxidation. What Is The Point Of The Electrode.
From jakenmedical.com
12Lead Resting EKG Electrode Placement Jaken Medical Inc What Is The Point Of The Electrode Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; There is a potential difference between the electrode and the electrolyte called the potential of the electrode. They’re the positively and negatively charged halves of the system. Electrodes are vital components of electrochemical cells. Each cell has two electrodes. Standard electrode potential is. What Is The Point Of The Electrode.
From www.researchgate.net
The electric arc causes melting of both electrode and the parent plate What Is The Point Of The Electrode Each cell has two electrodes. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrodes are vital components of electrochemical cells. Electrode, electric conductor, usually metal, used as either of the two terminals of an. What Is The Point Of The Electrode.
From saylordotorg.github.io
Standard Potentials What Is The Point Of The Electrode Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; They’re the positively and negatively charged halves of the system. Each cell has two electrodes. Standard electrode potential is a measurement of the potential for equilibrium. There is a potential difference between the electrode and the electrolyte called the potential of the electrode.. What Is The Point Of The Electrode.
From study.com
Electrode Definition, Types & Function Lesson What Is The Point Of The Electrode Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Each cell has two electrodes. Standard electrode potential is a measurement of the potential for equilibrium. They’re the positively and negatively charged halves of the system. There is a potential difference between the electrode and the electrolyte called the potential of the electrode.. What Is The Point Of The Electrode.
From saylordotorg.github.io
Electrochemistry What Is The Point Of The Electrode Electrodes are vital components of electrochemical cells. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode. What Is The Point Of The Electrode.
From www.youtube.com
What are different types of Reversible Electrodes? Electrochemistry What Is The Point Of The Electrode Standard electrode potential is a measurement of the potential for equilibrium. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode reaction refers to the net oxidation or reduction process that takes place at. What Is The Point Of The Electrode.
From chem.libretexts.org
11.2 Potentiometric Methods Chemistry LibreTexts What Is The Point Of The Electrode Standard electrode potential is a measurement of the potential for equilibrium. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; They’re the positively and negatively charged halves of the system. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Each cell has two electrodes.. What Is The Point Of The Electrode.
From www.researchgate.net
Schematics of threeelectrode cell, standard calomel electrode as What Is The Point Of The Electrode Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Electrodes are vital components of electrochemical cells. They’re the positively and negatively charged halves of the system. Standard electrode potential is a measurement of the potential for equilibrium. Each cell has two electrodes. There is a potential difference between the electrode and the. What Is The Point Of The Electrode.
From www.youtube.com
Explain the origin of single electrode potential? Electrochemistry What Is The Point Of The Electrode An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrodes are vital components of electrochemical cells. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Each cell has two electrodes. Standard electrode potential is a measurement of the potential for equilibrium. They’re the positively. What Is The Point Of The Electrode.
From www.doubtnut.com
Describe the construction and working of the calomel electrode. What Is The Point Of The Electrode An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Each cell has two electrodes. Electrodes are vital components of electrochemical cells. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrode, electric conductor, usually metal, used as either of the two terminals of an. What Is The Point Of The Electrode.
From chem.libretexts.org
1.7 Ion Selective Electrode Analysis Chemistry LibreTexts What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. They’re the positively and negatively charged halves of the system. Electrodes are vital components of electrochemical cells. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically. What Is The Point Of The Electrode.
From www.researchgate.net
Dimension of the circular electrode Download Scientific Diagram What Is The Point Of The Electrode There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Each cell has two electrodes. They’re the positively and negatively charged halves of the system. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Standard electrode potential is a measurement of the potential for equilibrium.. What Is The Point Of The Electrode.
From www.researchgate.net
Electrodes arrangement and used test cells a point electrode What Is The Point Of The Electrode They’re the positively and negatively charged halves of the system. Electrodes are vital components of electrochemical cells. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; An electrode reaction refers to the net oxidation or. What Is The Point Of The Electrode.
From www.researchgate.net
shows anatomical placement of the electrodes and the ECG signal What Is The Point Of The Electrode Each cell has two electrodes. Standard electrode potential is a measurement of the potential for equilibrium. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Electrodes are vital components of electrochemical cells. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; They’re the positively. What Is The Point Of The Electrode.
From www.researchgate.net
Fourpoint electrode configuration in a twolayer model of resistivity What Is The Point Of The Electrode Electrodes are vital components of electrochemical cells. Standard electrode potential is a measurement of the potential for equilibrium. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; There is a potential difference between the electrode. What Is The Point Of The Electrode.
From www.youtube.com
Electrochem Eng L0308 Polarization curve and example for an electrode What Is The Point Of The Electrode Each cell has two electrodes. They’re the positively and negatively charged halves of the system. Electrode, electric conductor, usually metal, used as either of the two terminals of an electrically conducting medium; Standard electrode potential is a measurement of the potential for equilibrium. An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode.. What Is The Point Of The Electrode.
From www.researchgate.net
Location scheme of the point electrode 1 a fragment of the part; 2 What Is The Point Of The Electrode An electrode reaction refers to the net oxidation or reduction process that takes place at an electrode. There is a potential difference between the electrode and the electrolyte called the potential of the electrode. Standard electrode potential is a measurement of the potential for equilibrium. They’re the positively and negatively charged halves of the system. Electrodes are vital components of. What Is The Point Of The Electrode.