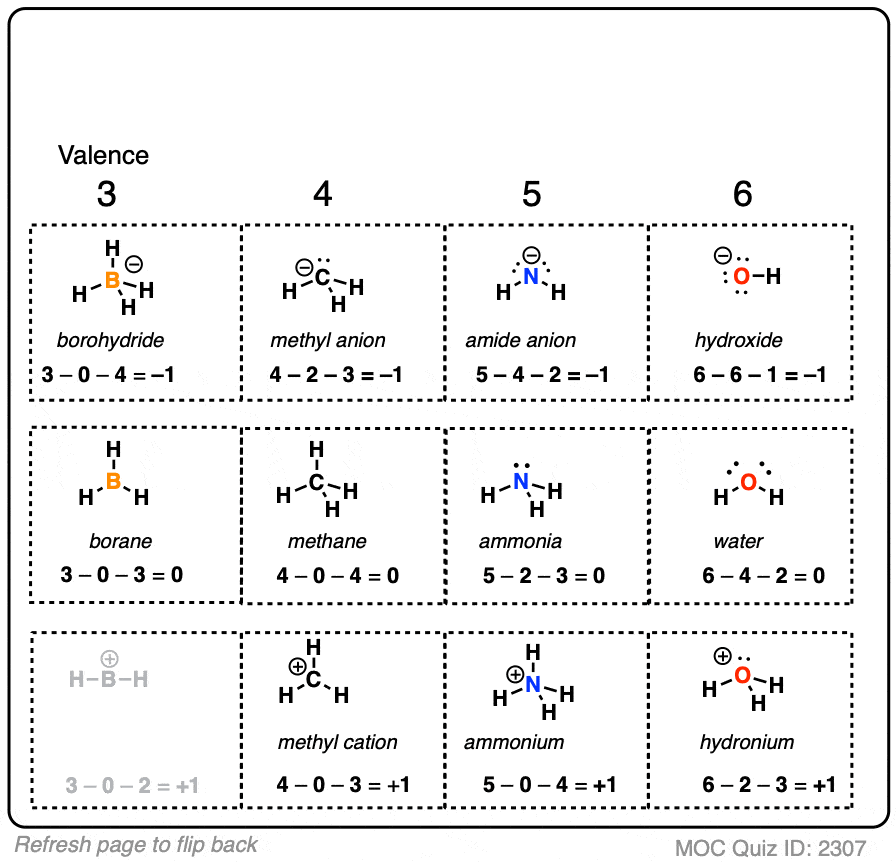
N20 Formal Charges . And given the example, there will be formal charge separation. For calculating the formal charge, you need to remember this formula; N 2 o lewis structure formal charge. Structure 1 is the most stable resonance because. For calculating the formal charge, you have to use. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o is a good example of. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. Structure 2 has some stability. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o.
from truyenhinhcapsongthu.net
Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. For calculating the formal charge, you need to remember this formula; N 2 o is a good example of. Structure 1 is the most stable resonance because. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. And given the example, there will be formal charge separation. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. For calculating the formal charge, you have to use. Structure 2 has some stability.
How To Calculate Formal Charge Master Organic Chemistry
N20 Formal Charges N 2 o lewis structure formal charge. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. For calculating the formal charge, you have to use. And given the example, there will be formal charge separation. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. For calculating the formal charge, you need to remember this formula; In order to calculate the formal charges for n2o we'll use the equation:formal charge =. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o is a good example of. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. Structure 1 is the most stable resonance because. Structure 2 has some stability. N 2 o lewis structure formal charge.
From byjus.com
Discuss resonance and formal charge in N_3^ and N_2O? N20 Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o lewis structure formal charge. Structure 2 has some stability. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. In short, now you have. N20 Formal Charges.
From www.youtube.com
N2O Molecular Geometry / Shape and Bond Angles YouTube N20 Formal Charges In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. And given the example, there will be formal charge separation. In order. N20 Formal Charges.
From www.numerade.com
SOLVED There are several possible Lewis structures for the molecule N20 Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. And given the example, there will be formal charge separation. Structure 2 has some stability. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. For calculating the. N20 Formal Charges.
From www.coursehero.com
[Solved] The molecule N2O, there are five possible Lewis Structures N20 Formal Charges N 2 o lewis structure formal charge. And given the example, there will be formal charge separation. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. The formal charge is defined. N20 Formal Charges.
From www.chegg.com
Species Resonance Structures Inchading Formal Charges N20 Formal Charges Structure 1 is the most stable resonance because. For calculating the formal charge, you need to remember this formula; The formal charge is defined as the charge over a particular molecule assuming that all the atoms. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o. N20 Formal Charges.
From www.youtube.com
How to Calculate the Formal Charges for N2O (Nitrous oxide or N20 Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o lewis structure formal charge. Structure 2 has some stability. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. And given the example, there will be formal. N20 Formal Charges.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry N20 Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o is a good example of. Structure 2 has some stability. And given the example, there will be formal charge separation. In short, now you have to find the formal charge on nitrogen (n) atoms. N20 Formal Charges.
From www.chegg.com
Solved 15. Choose the best Lewis structure for N20. N NFO A) N20 Formal Charges Structure 2 has some stability. For calculating the formal charge, you have to use. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. For calculating the formal charge, you need to remember this formula; And given the example, there will be formal charge separation. N 2 o is a good example of. N 2. N20 Formal Charges.
From www.masterorganicchemistry.com
How To Calculate Formal Charge N20 Formal Charges Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. Structure 1 is the most stable resonance because. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. Structure 2 has some stability. N 2. N20 Formal Charges.
From truyenhinhcapsongthu.net
How To Calculate Formal Charge Master Organic Chemistry N20 Formal Charges In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. For calculating the formal charge, you need to remember this formula; Structure 1 is the most stable resonance. N20 Formal Charges.
From iwantkowern.weebly.com
Calculating formal charge lewis structure iwantkowern N20 Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. For calculating the formal charge, you need to remember this formula; Structure 3 is the most unstable resonance. N20 Formal Charges.
From general.chemistrysteps.com
Formal Charges Chemistry Steps N20 Formal Charges Structure 2 has some stability. And given the example, there will be formal charge separation. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. For calculating the formal charge, you need to remember this formula; For calculating the formal charge, you have to use.. N20 Formal Charges.
From study.com
Formal Charge Formula How to Calculate Formal Charge Video & Lesson N20 Formal Charges Structure 2 has some stability. N 2 o is a good example of. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. You'll want to calculate the formal charges on each atom to make sure you have. N20 Formal Charges.
From www.makingmolecules.com
Formal Charges — Making Molecules N20 Formal Charges For calculating the formal charge, you need to remember this formula; In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. N 2 o is a good example of. N 2 o lewis structure formal charge. Structure 2 has some stability. Structure 1 is the. N20 Formal Charges.
From questions.kunduz.com
The formal charge is the "charge" an elem... Physical Chemistry N20 Formal Charges Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. N 2 o is a good example of. And given the example, there will be formal charge separation. For calculating the formal charge, you need to remember this formula; In short, now you have to find the formal charge on. N20 Formal Charges.
From www.answersarena.com
[Solved] Determine the formal charge of nitrogen in the s N20 Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. Structure 1 is the most stable resonance because. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. N 2 o is a good example of. N 2. N20 Formal Charges.
From www.masterorganicchemistry.com
How To Calculate Formal Charge N20 Formal Charges Structure 1 is the most stable resonance because. And given the example, there will be formal charge separation. For calculating the formal charge, you need to remember this formula; N 2 o is a good example of. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o.. N20 Formal Charges.
From www.organicchemistrytutor.com
Formal Charges — Organic Chemistry Tutor N20 Formal Charges Structure 1 is the most stable resonance because. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. And given the example, there will be formal charge separation.. N20 Formal Charges.
From aunitedkingdomfilm.com
Formal Charge N2o N20 Formal Charges For calculating the formal charge, you need to remember this formula; Structure 2 has some stability. Structure 1 is the most stable resonance because. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2 o is a good example of. N 2 o lewis structure formal charge. In order to calculate. N20 Formal Charges.
From www.slideserve.com
PPT FORMAL CHARGE PowerPoint Presentation, free download ID4196511 N20 Formal Charges In order to calculate the formal charges for n2o we'll use the equation:formal charge =. For calculating the formal charge, you have to use. For calculating the formal charge, you need to remember this formula; In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule.. N20 Formal Charges.
From aunitedkingdomfilm.com
Formal Charge N2o N20 Formal Charges For calculating the formal charge, you have to use. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. Structure 2 has some stability. For calculating the formal charge, you need to remember this formula; In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom. N20 Formal Charges.
From guweb2.gonzaga.edu
CHEM 101 Formal charge N20 Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. N 2 o lewis structure formal charge. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. In order to calculate the formal charges for n2o we'll use the equation:formal. N20 Formal Charges.
From brainly.com
which of the following lewis diagrams best represents the bonding in N20 Formal Charges N 2 o is a good example of. And given the example, there will be formal charge separation. For calculating the formal charge, you need to remember this formula; Structure 2 has some stability. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. The formal charge is defined as. N20 Formal Charges.
From www.studyxapp.com
assign formal charges to each atom in the resonance forms of n20 drag N20 Formal Charges And given the example, there will be formal charge separation. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. Structure 2 has some stability. N 2 o is a good example of. For calculating the formal charge, you have to use. In short, now you have to find the formal charge on. N20 Formal Charges.
From www.youtube.com
How to calculate the formal charge quickly YouTube N20 Formal Charges For calculating the formal charge, you need to remember this formula; In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. Structure 2 has some stability. The formal charge is defined as the charge over a particular molecule assuming that all the atoms. N 2. N20 Formal Charges.
From www.youtube.com
N2O Lewis Structure YouTube N20 Formal Charges N 2 o lewis structure formal charge. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. Structure 1 is the most stable resonance because. For calculating the formal charge, you have to use. The formal charge is defined as the charge over a particular molecule assuming that all the. N20 Formal Charges.
From www.expii.com
Formal Charge — Overview & Calculation Expii N20 Formal Charges You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. N 2 o lewis structure formal charge. Structure 1 is the most stable resonance because. And given. N20 Formal Charges.
From quizlet.com
What are the formal charges of \ce{N2O}? Quizlet N20 Formal Charges Structure 2 has some stability. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. Structure 1 is the most stable resonance because. For calculating the formal charge, you need to remember this formula; In order to calculate the formal charges for n2o we'll use the equation:formal charge =. N. N20 Formal Charges.
From www.chegg.com
Solved One of the Lewis structures that can be drawn for the N20 Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. And given the. N20 Formal Charges.
From www.collegesearch.in
Formal Charge Formula Definitions, Examples, Significance, Fun Facts N20 Formal Charges N 2 o is a good example of. For calculating the formal charge, you need to remember this formula; For calculating the formal charge, you have to use. And given the example, there will be formal charge separation. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. Structure 2 has some stability. Structure 3. N20 Formal Charges.
From www.chemistrylearner.com
Physical Chemistry Page 2 of 2 Chemistry Learner N20 Formal Charges The formal charge is defined as the charge over a particular molecule assuming that all the atoms. For calculating the formal charge, you need to remember this formula; In order to calculate the formal charges for n2o we'll use the equation:formal charge =. And given the example, there will be formal charge separation. N 2 o lewis structure formal charge.. N20 Formal Charges.
From www.chegg.com
Solved • Complete the following table [1] N20 Lewis N20 Formal Charges Structure 1 is the most stable resonance because. In order to calculate the formal charges for n2o we'll use the equation:formal charge =. In short, now you have to find the formal charge on nitrogen (n) atoms as well as oxygen (o) atom present in the n2o molecule. Structure 3 is the most unstable resonance of n 2 o because. N20 Formal Charges.
From clarkfvspears.blogspot.com
How to Calculate Formal Charge ClarkfvSpears N20 Formal Charges For calculating the formal charge, you need to remember this formula; N 2 o lewis structure formal charge. Structure 3 is the most unstable resonance of n 2 o because there is a positive charge on oxygen atom. N 2 o is a good example of. For calculating the formal charge, you have to use. And given the example, there. N20 Formal Charges.
From www.dailymotion.com
N2O Lewis Structure How to Draw the Lewis Structure for N2O video N20 Formal Charges For calculating the formal charge, you need to remember this formula; N 2 o is a good example of. For calculating the formal charge, you have to use. And given the example, there will be formal charge separation. N 2 o lewis structure formal charge. Structure 1 is the most stable resonance because. Structure 3 is the most unstable resonance. N20 Formal Charges.
From www.chegg.com
Solved QUESTION 7 Nitrous oxide, N20, is sometimes called N20 Formal Charges N 2 o is a good example of. Structure 2 has some stability. Structure 1 is the most stable resonance because. You'll want to calculate the formal charges on each atom to make sure you have the best lewis structure for n 2 o. The formal charge is defined as the charge over a particular molecule assuming that all the. N20 Formal Charges.