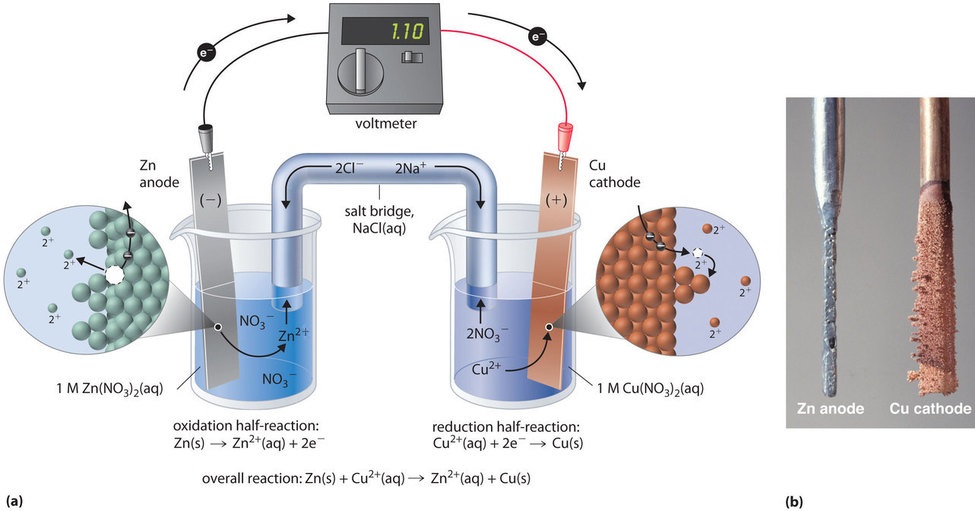
Real Life Applications Of Galvanic Cells . In principle, any galvanic cell could be used as a battery. The energy is harnessed by separating the. Nevertheless dry cells have a limited life. What are some of the applications of. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. A battery (storage cell) is a galvanic cell. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. Real batteries strike a balance between ideal characteristics and practical limitations. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell.
from chem.libretexts.org
In principle, any galvanic cell could be used as a battery. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. Nevertheless dry cells have a limited life. A battery (storage cell) is a galvanic cell. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. Real batteries strike a balance between ideal characteristics and practical limitations. The energy is harnessed by separating the. What are some of the applications of. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and.
2.1 Galvanic Cells Chemistry LibreTexts
Real Life Applications Of Galvanic Cells The energy is harnessed by separating the. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. The energy is harnessed by separating the. A battery (storage cell) is a galvanic cell. Nevertheless dry cells have a limited life. In principle, any galvanic cell could be used as a battery. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. What are some of the applications of. Real batteries strike a balance between ideal characteristics and practical limitations.
From webmis.highland.cc.il.us
Commercial Galvanic Cells Real Life Applications Of Galvanic Cells What are some of the applications of. In principle, any galvanic cell could be used as a battery. A battery (storage cell) is a galvanic cell. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy. Real Life Applications Of Galvanic Cells.
From studylib.net
Galvanic Cell Concept Real Life Applications Of Galvanic Cells Nevertheless dry cells have a limited life. Real batteries strike a balance between ideal characteristics and practical limitations. What are some of the applications of. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. In principle, any galvanic cell could be used as a battery. A battery. Real Life Applications Of Galvanic Cells.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID298076 Real Life Applications Of Galvanic Cells Real batteries strike a balance between ideal characteristics and practical limitations. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. What are some of the applications of. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. In principle, any galvanic cell could be. Real Life Applications Of Galvanic Cells.
From www.youtube.com
Galvanic cell or Voltaic cell Definition ,Construction and Working Real Life Applications Of Galvanic Cells An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. A battery (storage cell) is a galvanic cell. Real batteries strike a balance between ideal characteristics and practical limitations. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a. Real Life Applications Of Galvanic Cells.
From studylib.net
Applications of Galvanic Cells Real Life Applications Of Galvanic Cells One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. A battery (storage cell) is a galvanic cell. What are some of the applications of. In principle, any galvanic cell could be used as a battery.. Real Life Applications Of Galvanic Cells.
From www.slideserve.com
PPT Utilizes relationship between chemical potential energy Real Life Applications Of Galvanic Cells In principle, any galvanic cell could be used as a battery. Real batteries strike a balance between ideal characteristics and practical limitations. The energy is harnessed by separating the. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Voltaic cell (galvanic cell) is an electrochemical cell which. Real Life Applications Of Galvanic Cells.
From www.flickr.com
Гальванические процессы и их применение глория ключ 3уц Flickr Real Life Applications Of Galvanic Cells Nevertheless dry cells have a limited life. In principle, any galvanic cell could be used as a battery. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity.. Real Life Applications Of Galvanic Cells.
From www.youtube.com
Introduction to Galvanic Cells YouTube Real Life Applications Of Galvanic Cells What are some of the applications of. In principle, any galvanic cell could be used as a battery. Nevertheless dry cells have a limited life. A battery (storage cell) is a galvanic cell. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Galvanic cells have a wide. Real Life Applications Of Galvanic Cells.
From www.researchgate.net
Examples of two galvanic cells Download Scientific Diagram Real Life Applications Of Galvanic Cells Nevertheless dry cells have a limited life. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. The zinc electrode is eventually eaten away by the slightly acidic. Real Life Applications Of Galvanic Cells.
From school.careers360.com
galvanic cell Overview, Structure, Properties & Uses Real Life Applications Of Galvanic Cells The energy is harnessed by separating the. Real batteries strike a balance between ideal characteristics and practical limitations. In principle, any galvanic cell could be used as a battery. What are some of the applications of. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. A battery (storage cell). Real Life Applications Of Galvanic Cells.
From flatworldknowledge.lardbucket.org
Describing Electrochemical Cells Real Life Applications Of Galvanic Cells Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. Nevertheless dry cells have a. Real Life Applications Of Galvanic Cells.
From www.shutterstock.com
1,233 Galvanic Cells Images, Stock Photos & Vectors Shutterstock Real Life Applications Of Galvanic Cells Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat. Real Life Applications Of Galvanic Cells.
From www.collegesearch.in
Galvanic Cells Definitions, Examples, How They are Created, Principles Real Life Applications Of Galvanic Cells The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. Real batteries strike a balance between ideal characteristics and practical limitations. In principle, any galvanic cell could be used as a battery. A battery (storage cell) is a galvanic cell. Nevertheless dry cells have a limited life. What are some of the applications of. The energy is. Real Life Applications Of Galvanic Cells.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Real Life Applications Of Galvanic Cells The energy is harnessed by separating the. Nevertheless dry cells have a limited life. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. An ideal battery would never run down, produce an unchanging voltage, and be. Real Life Applications Of Galvanic Cells.
From www.youtube.com
Galvanic Cell Definition, Construction, Working, Example, Diagram Real Life Applications Of Galvanic Cells Nevertheless dry cells have a limited life. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Real batteries strike a balance between ideal characteristics and practical limitations. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and.. Real Life Applications Of Galvanic Cells.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Real Life Applications Of Galvanic Cells An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. A battery (storage cell) is a galvanic cell. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. What are some of the applications of. Nevertheless dry cells. Real Life Applications Of Galvanic Cells.
From medium.com
Galvanic Cells. In the world of electrochemistry… by Abdullah Medium Real Life Applications Of Galvanic Cells The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. Nevertheless dry cells have a limited life. The energy is harnessed by separating the. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. A battery (storage cell) is a galvanic cell. Real batteries strike a balance. Real Life Applications Of Galvanic Cells.
From www.youtube.com
Galvanic Cells Explained // Preliminary HSC Chemistry YouTube Real Life Applications Of Galvanic Cells A battery (storage cell) is a galvanic cell. Real batteries strike a balance between ideal characteristics and practical limitations. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. The energy is harnessed by separating the. One. Real Life Applications Of Galvanic Cells.
From cefvujil.blob.core.windows.net
A Galvanic Cell The Salt Bridge at John Mackay blog Real Life Applications Of Galvanic Cells Real batteries strike a balance between ideal characteristics and practical limitations. What are some of the applications of. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. Nevertheless dry cells have a limited life. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox. Real Life Applications Of Galvanic Cells.
From www.youtube.com
CHEM 1180 Galvanic Cells and Activity Series Lab YouTube Real Life Applications Of Galvanic Cells The energy is harnessed by separating the. Nevertheless dry cells have a limited life. A battery (storage cell) is a galvanic cell. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras,. Real Life Applications Of Galvanic Cells.
From studiousguy.com
Examples of Galvanic Cells in Everyday Life StudiousGuy Real Life Applications Of Galvanic Cells The energy is harnessed by separating the. In principle, any galvanic cell could be used as a battery. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. A battery (storage cell) is a galvanic cell. What are some of the applications of. One of the most common. Real Life Applications Of Galvanic Cells.
From chem.libretexts.org
Electrolytic Cells Chemistry LibreTexts Real Life Applications Of Galvanic Cells An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. In principle, any galvanic cell could be used as a battery. A battery (storage cell) is a. Real Life Applications Of Galvanic Cells.
From joiujlkil.blob.core.windows.net
Standard Cell Potential Galvanic at Rhonda Beecher blog Real Life Applications Of Galvanic Cells An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. A battery (storage cell). Real Life Applications Of Galvanic Cells.
From chem.libretexts.org
2.1 Galvanic Cells Chemistry LibreTexts Real Life Applications Of Galvanic Cells What are some of the applications of. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. In principle, any galvanic cell could be used as a battery. A battery (storage cell) is a galvanic cell. An ideal battery would never run down, produce an unchanging voltage, and be capable. Real Life Applications Of Galvanic Cells.
From www.youtube.com
Galvanic Cell YouTube Real Life Applications Of Galvanic Cells Nevertheless dry cells have a limited life. In principle, any galvanic cell could be used as a battery. What are some of the applications of. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. A battery (storage cell) is a galvanic cell. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous. Real Life Applications Of Galvanic Cells.
From courses.lumenlearning.com
Galvanic Cells General Chemistry Real Life Applications Of Galvanic Cells Nevertheless dry cells have a limited life. A battery (storage cell) is a galvanic cell. What are some of the applications of. Real batteries strike a balance between ideal characteristics and practical limitations. In principle, any galvanic cell could be used as a battery. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls,. Real Life Applications Of Galvanic Cells.
From psu.pb.unizin.org
Galvanic Cells (17.2) Chemistry 110 Real Life Applications Of Galvanic Cells What are some of the applications of. The energy is harnessed by separating the. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. Nevertheless dry cells have a limited life. The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. Real batteries strike a balance. Real Life Applications Of Galvanic Cells.
From electrochemistryh5t34.blogspot.kr
We Love Electrochemistry Galvanic Cell Real Life Applications Of Galvanic Cells The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Real batteries strike a balance between ideal characteristics and practical limitations. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous. Real Life Applications Of Galvanic Cells.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Real Life Applications Of Galvanic Cells An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. The energy is harnessed by separating the. The zinc electrode is eventually eaten away by the slightly acidic. Real Life Applications Of Galvanic Cells.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Real Life Applications Of Galvanic Cells Real batteries strike a balance between ideal characteristics and practical limitations. A battery (storage cell) is a galvanic cell. In principle, any galvanic cell could be used as a battery. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. Galvanic cells have a wide variety of applications, and. Real Life Applications Of Galvanic Cells.
From www.youtube.com
Galvanic Cells YouTube Real Life Applications Of Galvanic Cells Nevertheless dry cells have a limited life. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. The energy is harnessed by separating the. A battery (storage cell) is a. Real Life Applications Of Galvanic Cells.
From www1.chem.umn.edu
CopperZinc Galvanic Cell Real Life Applications Of Galvanic Cells In principle, any galvanic cell could be used as a battery. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones, cameras, laptops, and. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. The energy is harnessed by separating the.. Real Life Applications Of Galvanic Cells.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID3222668 Real Life Applications Of Galvanic Cells The zinc electrode is eventually eaten away by the slightly acidic ammonium chloride. What are some of the applications of. Real batteries strike a balance between ideal characteristics and practical limitations. A battery (storage cell) is a galvanic cell. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell.. Real Life Applications Of Galvanic Cells.
From studiousguy.com
Examples of Galvanic Cells in Everyday Life StudiousGuy Real Life Applications Of Galvanic Cells An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. One of the most common examples of an electrochemical cell (galvanic cell) used in our daily life is a \(1.5\;{\rm{v}}\) cell. Galvanic cells have a wide variety of applications, and are used in watches, clocks, remote controls, phones,. Real Life Applications Of Galvanic Cells.
From www.youtube.com
Galvanic Cell Example Daniell Cell YouTube Real Life Applications Of Galvanic Cells What are some of the applications of. An ideal battery would never run down, produce an unchanging voltage, and be capable of withstanding environmental extremes of heat and humidity. Voltaic cell (galvanic cell) is an electrochemical cell which derives electrical energy from spontaneous redox reactions occurring in the cell. One of the most common examples of an electrochemical cell (galvanic. Real Life Applications Of Galvanic Cells.