What Temperature Does Ice Evaporate . A mixture of ice and water, the water will. Dry ice is actually solid, frozen carbon dioxide, which happens to. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. what are freezing and melting? Melting ice cubes illustrate the process of this phase. the melting point of ice is 0°c. when a solid like my ice lolly is heated, it melts to become a liquid. Find out about these reversible processes. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. a container holding dry ice (frozen carbon dioxide) sublimating into the air. As the ice heats up, it melts and turns into water. And if you heat up a liquid even more, it’ll evaporate to. When solid water is exposed to enough heat, it will melt.
from scitechdaily.com
Find out about these reversible processes. And if you heat up a liquid even more, it’ll evaporate to. A mixture of ice and water, the water will. Melting ice cubes illustrate the process of this phase. When solid water is exposed to enough heat, it will melt. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. As the ice heats up, it melts and turns into water. Dry ice is actually solid, frozen carbon dioxide, which happens to. what are freezing and melting? when a solid like my ice lolly is heated, it melts to become a liquid.
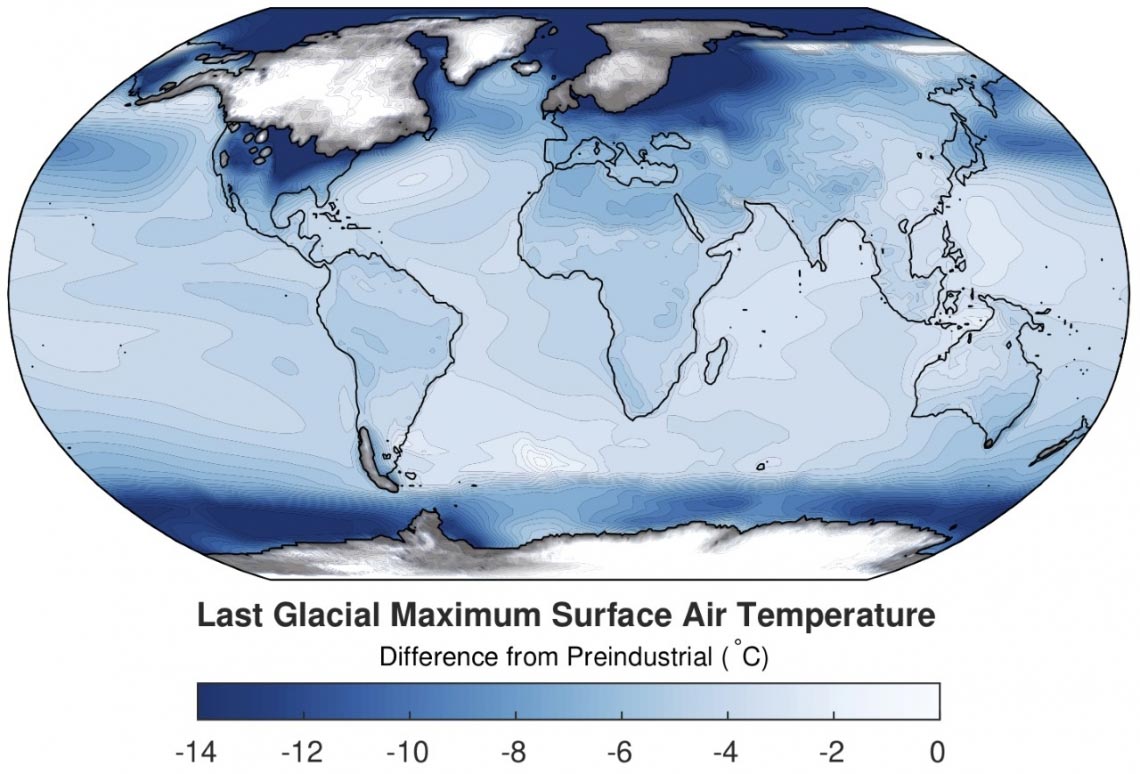
How Cold Was the Last Ice Age? Researchers Have Now Mapped the
What Temperature Does Ice Evaporate As the ice heats up, it melts and turns into water. a container holding dry ice (frozen carbon dioxide) sublimating into the air. Melting ice cubes illustrate the process of this phase. when a solid like my ice lolly is heated, it melts to become a liquid. A mixture of ice and water, the water will. the melting point of ice is 0°c. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. what are freezing and melting? Dry ice is actually solid, frozen carbon dioxide, which happens to. And if you heat up a liquid even more, it’ll evaporate to. Find out about these reversible processes. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. When solid water is exposed to enough heat, it will melt. As the ice heats up, it melts and turns into water.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? What Temperature Does Ice Evaporate a container holding dry ice (frozen carbon dioxide) sublimating into the air. As the ice heats up, it melts and turns into water. When solid water is exposed to enough heat, it will melt. Find out about these reversible processes. And if you heat up a liquid even more, it’ll evaporate to. what are freezing and melting? . What Temperature Does Ice Evaporate.
From study.com
Polar Ice Caps Melting Causes & Impacts Lesson What Temperature Does Ice Evaporate And if you heat up a liquid even more, it’ll evaporate to. Dry ice is actually solid, frozen carbon dioxide, which happens to. When solid water is exposed to enough heat, it will melt. a container holding dry ice (frozen carbon dioxide) sublimating into the air. when liquid water reaches a low enough temperature, it freezes and becomes. What Temperature Does Ice Evaporate.
From slideplayer.com
WarmUp Think critically What do you think is happening to the What Temperature Does Ice Evaporate Find out about these reversible processes. When solid water is exposed to enough heat, it will melt. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. a container holding dry ice (frozen carbon dioxide) sublimating into the air. what are freezing and melting? As the ice heats up, it melts and turns. What Temperature Does Ice Evaporate.
From www.artofit.org
At what temperature does ice melt work 10 examples Artofit What Temperature Does Ice Evaporate what are freezing and melting? And if you heat up a liquid even more, it’ll evaporate to. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. Melting ice cubes illustrate the process of this phase. When solid water is exposed to enough heat, it will melt. Dry ice is actually solid, frozen carbon. What Temperature Does Ice Evaporate.
From safethaw.com
At What Temperature Does Ice Melt? Factors That Influence Ice Melting What Temperature Does Ice Evaporate when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. And if you heat up a liquid even more, it’ll evaporate to. Find out about these reversible processes. Melting ice cubes illustrate the process of this phase. When solid water is exposed to enough heat, it will melt. although much less studied than the. What Temperature Does Ice Evaporate.
From www.youtube.com
What temperature does ice cream melt? YouTube What Temperature Does Ice Evaporate And if you heat up a liquid even more, it’ll evaporate to. a container holding dry ice (frozen carbon dioxide) sublimating into the air. what are freezing and melting? the melting point of ice is 0°c. As the ice heats up, it melts and turns into water. Dry ice is actually solid, frozen carbon dioxide, which happens. What Temperature Does Ice Evaporate.
From www.youtube.com
what is the final temperature when ice is added to water? YouTube What Temperature Does Ice Evaporate what are freezing and melting? Melting ice cubes illustrate the process of this phase. And if you heat up a liquid even more, it’ll evaporate to. a container holding dry ice (frozen carbon dioxide) sublimating into the air. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. When. What Temperature Does Ice Evaporate.
From stock.adobe.com
Change of State Water. State of matter. Change of water according to What Temperature Does Ice Evaporate And if you heat up a liquid even more, it’ll evaporate to. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. A mixture of ice and water, the water will. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. Melting ice cubes illustrate the. What Temperature Does Ice Evaporate.
From www.youtube.com
Final Temperature of Ice and Water Mixture How Many Grams of Ice Will What Temperature Does Ice Evaporate When solid water is exposed to enough heat, it will melt. the melting point of ice is 0°c. Find out about these reversible processes. A mixture of ice and water, the water will. And if you heat up a liquid even more, it’ll evaporate to. although much less studied than the evaporation of liquids, the sublimation of solid. What Temperature Does Ice Evaporate.
From safethaw.com
Exploring The Science What Temperature Does Ice Melt At What Temperature Does Ice Evaporate Dry ice is actually solid, frozen carbon dioxide, which happens to. a container holding dry ice (frozen carbon dioxide) sublimating into the air. As the ice heats up, it melts and turns into water. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. Find out about these reversible processes. what are freezing. What Temperature Does Ice Evaporate.
From tractionmagic.com
Understanding The Melting Point At What Temperature Does Ice Melt? What Temperature Does Ice Evaporate As the ice heats up, it melts and turns into water. what are freezing and melting? Dry ice is actually solid, frozen carbon dioxide, which happens to. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. When solid water is exposed to enough heat, it will melt. Melting ice. What Temperature Does Ice Evaporate.
From safepaw.com
What Temperature Does Salt Melt Ice? Science Behind Salt and Ice What Temperature Does Ice Evaporate when a solid like my ice lolly is heated, it melts to become a liquid. A mixture of ice and water, the water will. a container holding dry ice (frozen carbon dioxide) sublimating into the air. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. when liquid. What Temperature Does Ice Evaporate.
From safepaw.com
What Temperature Does Salt Melt Ice? Science Behind Salt and Ice What Temperature Does Ice Evaporate A mixture of ice and water, the water will. Find out about these reversible processes. a container holding dry ice (frozen carbon dioxide) sublimating into the air. Melting ice cubes illustrate the process of this phase. And if you heat up a liquid even more, it’ll evaporate to. the melting point of ice is 0°c. When solid water. What Temperature Does Ice Evaporate.
From safethaw.com
At What Temperature Does Ice Melt Factors & Melting Point Differences What Temperature Does Ice Evaporate Find out about these reversible processes. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. when a solid like my ice lolly is heated, it melts to become a liquid. And if you heat up a liquid even more, it’ll evaporate to. the melting point of ice is 0°c. A mixture of. What Temperature Does Ice Evaporate.
From scitechdaily.com
How Cold Was the Last Ice Age? Researchers Have Now Mapped the What Temperature Does Ice Evaporate although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. when a solid like my ice lolly is heated, it melts to become a liquid. Melting ice cubes illustrate the process of this phase. a container holding dry ice (frozen carbon dioxide) sublimating into the air. what are. What Temperature Does Ice Evaporate.
From www.exploratorium.edu
Global Climate Change Explorer Ice Exploratorium What Temperature Does Ice Evaporate the melting point of ice is 0°c. what are freezing and melting? Dry ice is actually solid, frozen carbon dioxide, which happens to. Find out about these reversible processes. a container holding dry ice (frozen carbon dioxide) sublimating into the air. And if you heat up a liquid even more, it’ll evaporate to. although much less. What Temperature Does Ice Evaporate.
From tractionmagic.com
Understanding The Melting Point At What Temperature Does Ice Melt? What Temperature Does Ice Evaporate when a solid like my ice lolly is heated, it melts to become a liquid. Melting ice cubes illustrate the process of this phase. Dry ice is actually solid, frozen carbon dioxide, which happens to. the melting point of ice is 0°c. And if you heat up a liquid even more, it’ll evaporate to. A mixture of ice. What Temperature Does Ice Evaporate.
From www.numerade.com
SOLVED Dry ice, CO2(s), does not melt or freeze at atmospheric What Temperature Does Ice Evaporate a container holding dry ice (frozen carbon dioxide) sublimating into the air. what are freezing and melting? the melting point of ice is 0°c. Melting ice cubes illustrate the process of this phase. Dry ice is actually solid, frozen carbon dioxide, which happens to. Find out about these reversible processes. And if you heat up a liquid. What Temperature Does Ice Evaporate.
From safethaw.com
Understanding Ice Melting At What Temperature Does Ice Begin to Melt What Temperature Does Ice Evaporate when a solid like my ice lolly is heated, it melts to become a liquid. what are freezing and melting? A mixture of ice and water, the water will. Melting ice cubes illustrate the process of this phase. Dry ice is actually solid, frozen carbon dioxide, which happens to. a container holding dry ice (frozen carbon dioxide). What Temperature Does Ice Evaporate.
From safethaw.com
At What Temperature Does Ice Melt? A Comprehensive Exploration Of The What Temperature Does Ice Evaporate When solid water is exposed to enough heat, it will melt. Dry ice is actually solid, frozen carbon dioxide, which happens to. what are freezing and melting? although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. a container holding dry ice (frozen carbon dioxide) sublimating into the air.. What Temperature Does Ice Evaporate.
From safethaw.com
What Temperature Does Ice Melt? Uncovering The Science Behind Melting What Temperature Does Ice Evaporate what are freezing and melting? And if you heat up a liquid even more, it’ll evaporate to. when a solid like my ice lolly is heated, it melts to become a liquid. a container holding dry ice (frozen carbon dioxide) sublimating into the air. When solid water is exposed to enough heat, it will melt. Melting ice. What Temperature Does Ice Evaporate.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition What Temperature Does Ice Evaporate a container holding dry ice (frozen carbon dioxide) sublimating into the air. Melting ice cubes illustrate the process of this phase. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. Find out about these. What Temperature Does Ice Evaporate.
From www.metoffice.gov.uk
Sea ice in the climate system Met Office What Temperature Does Ice Evaporate When solid water is exposed to enough heat, it will melt. Find out about these reversible processes. And if you heat up a liquid even more, it’ll evaporate to. Melting ice cubes illustrate the process of this phase. As the ice heats up, it melts and turns into water. when a solid like my ice lolly is heated, it. What Temperature Does Ice Evaporate.
From pote.com
Extreme Ice Cream Cook's Science What Temperature Does Ice Evaporate A mixture of ice and water, the water will. the melting point of ice is 0°c. when a solid like my ice lolly is heated, it melts to become a liquid. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. what are freezing and melting? When solid. What Temperature Does Ice Evaporate.
From dxoustrzl.blob.core.windows.net
What Temp Does Ice Melt Work At at Daniel Auten blog What Temperature Does Ice Evaporate Dry ice is actually solid, frozen carbon dioxide, which happens to. As the ice heats up, it melts and turns into water. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. And if you heat up a liquid even more, it’ll evaporate to. although much less studied than the evaporation of liquids, the. What Temperature Does Ice Evaporate.
From socratic.org
What is the profile of the graph of temperature versus time, when water What Temperature Does Ice Evaporate Dry ice is actually solid, frozen carbon dioxide, which happens to. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. Melting ice cubes illustrate the process of this phase. And if you heat up a liquid even more, it’ll evaporate to. what are freezing and melting? A mixture of ice and water, the. What Temperature Does Ice Evaporate.
From primaryleap.co.uk
Chemistry States Of Matter Level 2 activity for kids PrimaryLeap.co.uk What Temperature Does Ice Evaporate A mixture of ice and water, the water will. a container holding dry ice (frozen carbon dioxide) sublimating into the air. Find out about these reversible processes. When solid water is exposed to enough heat, it will melt. what are freezing and melting? And if you heat up a liquid even more, it’ll evaporate to. when a. What Temperature Does Ice Evaporate.
From safethaw.com
At What Temperature Does Ice Melt? A Comprehensive Exploration Of The What Temperature Does Ice Evaporate although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. what are freezing and melting? when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. Melting ice cubes illustrate the process of this phase. A mixture of ice and water, the water will. when. What Temperature Does Ice Evaporate.
From www.numerade.com
The temperature of a 100 g piece of ice is risen steadily so that the What Temperature Does Ice Evaporate what are freezing and melting? when a solid like my ice lolly is heated, it melts to become a liquid. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. the melting point of ice is 0°c. A mixture of ice and water, the water will. although much less studied than. What Temperature Does Ice Evaporate.
From dxoustrzl.blob.core.windows.net
What Temp Does Ice Melt Work At at Daniel Auten blog What Temperature Does Ice Evaporate Find out about these reversible processes. when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. although much less studied than the evaporation of liquids, the sublimation of solid ice has important consequences, as. Dry ice is actually solid, frozen carbon dioxide, which happens to. the melting point of ice is 0°c. A. What Temperature Does Ice Evaporate.
From www.britannica.com
Hydrosphere Definition, Layers, Examples, & Facts Britannica What Temperature Does Ice Evaporate when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. a container holding dry ice (frozen carbon dioxide) sublimating into the air. Dry ice is actually solid, frozen carbon dioxide, which happens to. when a solid like my ice lolly is heated, it melts to become a liquid. the melting point of. What Temperature Does Ice Evaporate.
From safethaw.com
The Science of Ice Melting What Temperature Will Guarantee It? What Temperature Does Ice Evaporate And if you heat up a liquid even more, it’ll evaporate to. As the ice heats up, it melts and turns into water. A mixture of ice and water, the water will. what are freezing and melting? when a solid like my ice lolly is heated, it melts to become a liquid. Find out about these reversible processes.. What Temperature Does Ice Evaporate.
From safepaw.com
What temperature does salt and ice melt work? Safe Paw What Temperature Does Ice Evaporate the melting point of ice is 0°c. when a solid like my ice lolly is heated, it melts to become a liquid. Find out about these reversible processes. what are freezing and melting? When solid water is exposed to enough heat, it will melt. And if you heat up a liquid even more, it’ll evaporate to. Melting. What Temperature Does Ice Evaporate.
From temperaturemaster.com
Exploring the Evaporation Process of Dry Ice What Temperature Does Ice Evaporate And if you heat up a liquid even more, it’ll evaporate to. what are freezing and melting? when liquid water reaches a low enough temperature, it freezes and becomes a solid—ice. Dry ice is actually solid, frozen carbon dioxide, which happens to. Melting ice cubes illustrate the process of this phase. As the ice heats up, it melts. What Temperature Does Ice Evaporate.
From socratic.org
Does dry ice melt or evaporate? Socratic What Temperature Does Ice Evaporate a container holding dry ice (frozen carbon dioxide) sublimating into the air. when a solid like my ice lolly is heated, it melts to become a liquid. A mixture of ice and water, the water will. Dry ice is actually solid, frozen carbon dioxide, which happens to. And if you heat up a liquid even more, it’ll evaporate. What Temperature Does Ice Evaporate.