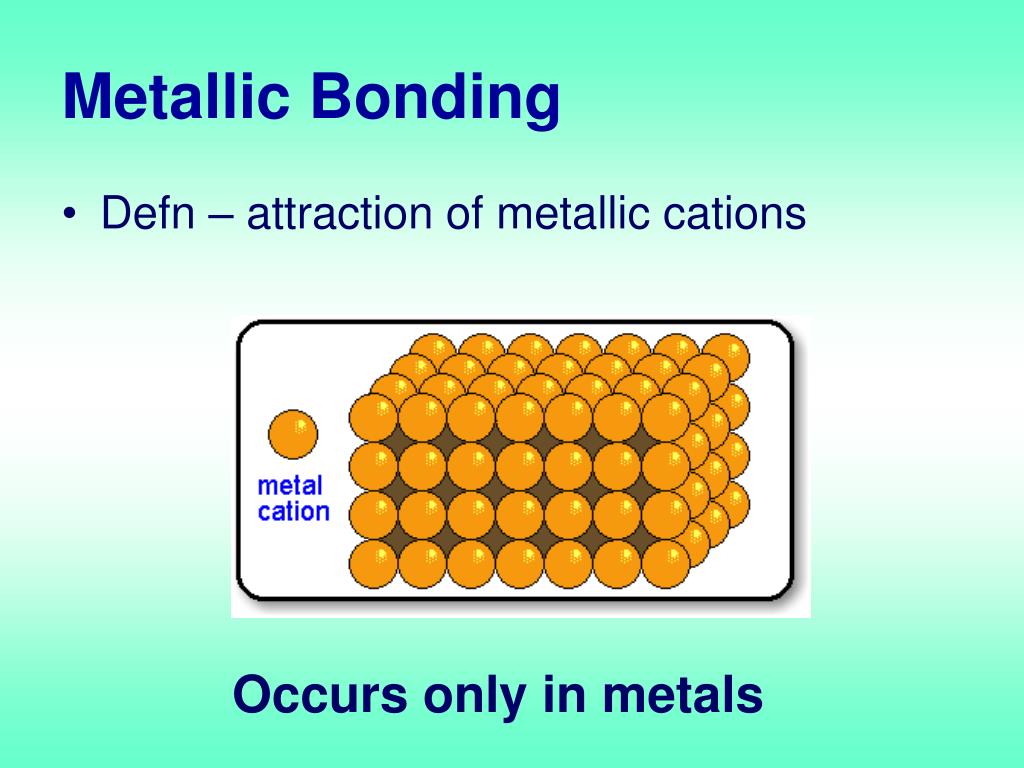
Bonding In Metal . In contrast, covalent and ionic bonds form between two discrete atoms. Metallic bond, force that holds atoms together in a metallic substance. Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. Name some physical properties of metals that reflect this difference. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. These free electrons are called delocalized because they are not confined (localized) to one atom. Metallic bonding is the main type of chemical bond that forms between metal atoms. Metallic bonds occur among metal atoms. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. A sheet of aluminum foil and a copper wire are both places where you can see metallic
from www.slideserve.com
Metallic bonding is the main type of chemical bond that forms between metal atoms. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Metallic bonds occur among metal atoms. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. A sheet of aluminum foil and a copper wire are both places where you can see metallic Metallic bond, force that holds atoms together in a metallic substance. Name some physical properties of metals that reflect this difference.
PPT Chemical Bonding PowerPoint Presentation, free download ID4982303
Bonding In Metal Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. These free electrons are called delocalized because they are not confined (localized) to one atom. Name some physical properties of metals that reflect this difference. Metallic bonding is the main type of chemical bond that forms between metal atoms. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Metallic bonds occur among metal atoms. Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bond, force that holds atoms together in a metallic substance. A sheet of aluminum foil and a copper wire are both places where you can see metallic In contrast, covalent and ionic bonds form between two discrete atoms.
From cronodon.com
Metals Bonding In Metal In contrast, covalent and ionic bonds form between two discrete atoms. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Name some physical properties of metals that reflect this difference. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing.. Bonding In Metal.
From www.thesciencehive.co.uk
Metallic Bonding (GCSE) — the science sauce Bonding In Metal Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. A metallic bond is a type of chemical bond formed between positively charged. Bonding In Metal.
From www.tec-science.com
Metallic bonding tecscience Bonding In Metal The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared. Bonding In Metal.
From mungfali.com
Metallic Bonding Labelled Diagram Bonding In Metal Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. These free electrons are called delocalized because they are not confined (localized) to one atom. Metallic bonding is the main type of chemical bond that forms between metal atoms. Explain the fundamental difference between the bonding in metallic solids compared to that in other types. Bonding In Metal.
From www.expii.com
Metallic Bond — Formation & Compounds Expii Bonding In Metal Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Metallic bonds occur among metal atoms. Explain the fundamental difference between the bonding in metallic solids compared to that. Bonding In Metal.
From blog.thepipingmart.com
Metallic Bonding in Zinc An Overview Bonding In Metal Name some physical properties of metals that reflect this difference. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bonding is the main type of chemical bond. Bonding In Metal.
From www.tec-science.com
Lattice structure of metals tecscience Bonding In Metal Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. Metallic bonding is a type of chemical bonding where metal nuclei share free. Bonding In Metal.
From stock.adobe.com
Metallic bond Metallic bonding is the electrostatic attractive force Bonding In Metal These free electrons are called delocalized because they are not confined (localized) to one atom. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one. Bonding In Metal.
From www.youtube.com
Metallic Bonding and the Electron Sea Model, Electrical Conductivity Bonding In Metal In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and. Bonding In Metal.
From www.slideserve.com
PPT Ionic, Covalent and Metallic Bonding PowerPoint Presentation Bonding In Metal Name some physical properties of metals that reflect this difference. A sheet of aluminum foil and a copper wire are both places where you can see metallic Metallic bonds occur among metal atoms. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Metallic. Bonding In Metal.
From www.youtube.com
Metallic Bonding Animation YouTube Bonding In Metal A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bond, force that holds atoms together in a metallic substance. Name some physical properties of metals that reflect. Bonding In Metal.
From discovertutoring.co.uk
Metallic Bonding Explained Discover Tutoring Bonding In Metal Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. Metallic bonding is the main type of chemical bond that forms between metal atoms. Name some physical properties of metals that reflect this difference. Metallic bond, force that holds atoms together in a metallic substance. These free electrons are. Bonding In Metal.
From cupsoguepictures.com
😎 Metallic bonding in sodium. Metallic bonding in Potassium?. 20190216 Bonding In Metal Metallic bonding is the main type of chemical bond that forms between metal atoms. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Metallic bonds occur among metal atoms. A sheet of aluminum foil and a copper wire are both places. Bonding In Metal.
From studymind.co.uk
Metallic Bonds (GCSE Chemistry) Study Mind Bonding In Metal These free electrons are called delocalized because they are not confined (localized) to one atom. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. Metallic bonding is the main type of chemical bond that forms between metal atoms. A sheet of aluminum foil. Bonding In Metal.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID4982303 Bonding In Metal In contrast, covalent and ionic bonds form between two discrete atoms. These free electrons are called delocalized because they are not confined (localized) to one atom. Metallic bond, force that holds atoms together in a metallic substance. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Explain the fundamental difference between the bonding in metallic solids. Bonding In Metal.
From www.thoughtco.com
Definition and Properties of Metallic Bonding Bonding In Metal These free electrons are called delocalized because they are not confined (localized) to one atom. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. In contrast, covalent and ionic bonds form between two discrete atoms. Metallic bonding is the main type of chemical. Bonding In Metal.
From revisechemistry.uk
Bonding and Structure Edexcel T1 revisechemistry.uk Bonding In Metal These free electrons are called delocalized because they are not confined (localized) to one atom. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. A sheet of aluminum foil and a copper wire are both places where you can see metallic Metallic bonds. Bonding In Metal.
From mungfali.com
Metallic Bonding Labelled Diagram Bonding In Metal In contrast, covalent and ionic bonds form between two discrete atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic Metallic bonding is the main type of chemical bond that forms between metal atoms. Name some physical properties of metals that reflect this difference. Metallic bond, force that holds atoms together in. Bonding In Metal.
From www.colourbox.com
Types of chemical bonding diagram. Stock vector Colourbox Bonding In Metal A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. A sheet of aluminum foil and a copper wire are both places where you can see metallic Metallic bond, force that holds atoms together in a metallic substance. In contrast, covalent and ionic bonds. Bonding In Metal.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Periodicity (2) Melting and boiling points of the Bonding In Metal Metallic bonds occur among metal atoms. Name some physical properties of metals that reflect this difference. Metallic bond, force that holds atoms together in a metallic substance. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near. Bonding In Metal.
From www.slideserve.com
PPT Unit 4 Metallic Bonding PowerPoint Presentation, free download Bonding In Metal A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. A sheet of aluminum foil and a copper wire are both places where you can see metallic Name some physical properties of metals that reflect this difference. In contrast, valence electrons are shared between. Bonding In Metal.
From www.science-revision.co.uk
Metallic bonding Bonding In Metal These free electrons are called delocalized because they are not confined (localized) to one atom. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one. Bonding In Metal.
From mungfali.com
Metallic Bonding Labelled Diagram Bonding In Metal Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. In contrast, covalent and ionic bonds form between two discrete atoms. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Explain the fundamental difference between the bonding in. Bonding In Metal.
From www.youtube.com
Chemistry 4.3 Metallic Bonding YouTube Bonding In Metal Metallic bonds occur among metal atoms. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice of cations. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bonding is the main type of chemical bond that forms between metal. Bonding In Metal.
From www.slideserve.com
PPT Metallic bonding and properties PowerPoint Presentation, free Bonding In Metal The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Metallic bond, force that holds atoms together in a metallic substance. In contrast, covalent and ionic bonds form between two discrete atoms. These free electrons are called delocalized because they are not confined (localized) to one atom. Metallic bonding is a type of chemical bonding where metal. Bonding In Metal.
From www.pinterest.co.kr
Metallic bonding vector illustration diagram Metallic bonding Bonding In Metal Name some physical properties of metals that reflect this difference. Metallic bond, force that holds atoms together in a metallic substance. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. These free electrons are called delocalized because they are not confined (localized) to one atom. A metallic bond is a type of chemical bond. Bonding In Metal.
From mungfali.com
Metallic Bonding Labelled Diagram Bonding In Metal These free electrons are called delocalized because they are not confined (localized) to one atom. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bonds occur among metal atoms. A sheet of aluminum foil and a copper wire are both places where you can see metallic Explain the fundamental difference between the bonding. Bonding In Metal.
From edu.rsc.org
How to teach metallic bonding Poster RSC Education Bonding In Metal A sheet of aluminum foil and a copper wire are both places where you can see metallic Explain the fundamental difference between the bonding in metallic solids compared to that in other types of solids and within molecules. In contrast, covalent and ionic bonds form between two discrete atoms. These free electrons are called delocalized because they are not confined. Bonding In Metal.
From www.breakingatom.com
Metallic Bonding Bonding In Metal Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bond, force that holds atoms together in a metallic substance. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. In contrast, covalent and ionic bonds form between two discrete atoms. A sheet of aluminum foil and a copper wire are. Bonding In Metal.
From www.britannica.com
chemical bonding Definition, Types, & Examples Britannica Bonding In Metal Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bond, force that holds atoms together in a metallic substance. In contrast, covalent and ionic bonds form between two discrete atoms. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Explain the fundamental difference between the bonding in metallic solids. Bonding In Metal.
From www.tec-science.com
Metallic bonding tecscience Bonding In Metal In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. Name some physical properties of metals that reflect this difference. Metallic bonding is a type of chemical bonding where metal nuclei share free valence electrons. Metallic bond, force that holds atoms together in a. Bonding In Metal.
From www.britannica.com
Metallic bond Properties, Examples, & Explanation Britannica Bonding In Metal Name some physical properties of metals that reflect this difference. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons are shared among a lattice. Bonding In Metal.
From www.science-revision.co.uk
Metallic bonding Bonding In Metal Name some physical properties of metals that reflect this difference. In contrast, valence electrons are shared between two atoms in a covalent bond and spend more time near one atom than the other in an ionic. These free electrons are called delocalized because they are not confined (localized) to one atom. Metallic bonding is the main type of chemical bond. Bonding In Metal.
From studyrocket.co.uk
Metallic Bonding GCSE Chemistry Science) AQA Revision Bonding In Metal A sheet of aluminum foil and a copper wire are both places where you can see metallic Metallic bonds occur among metal atoms. Name some physical properties of metals that reflect this difference. The outermost electron shell of each atom overlaps with many adjacent atoms, allowing. Metallic bond, force that holds atoms together in a metallic substance. In contrast, covalent. Bonding In Metal.
From www.slideshare.net
Metallic bonding Bonding In Metal Metallic bonding is the main type of chemical bond that forms between metal atoms. Name some physical properties of metals that reflect this difference. Metallic bonds occur among metal atoms. Metallic bond, force that holds atoms together in a metallic substance. A metallic bond is a type of chemical bond formed between positively charged atoms in which the free electrons. Bonding In Metal.