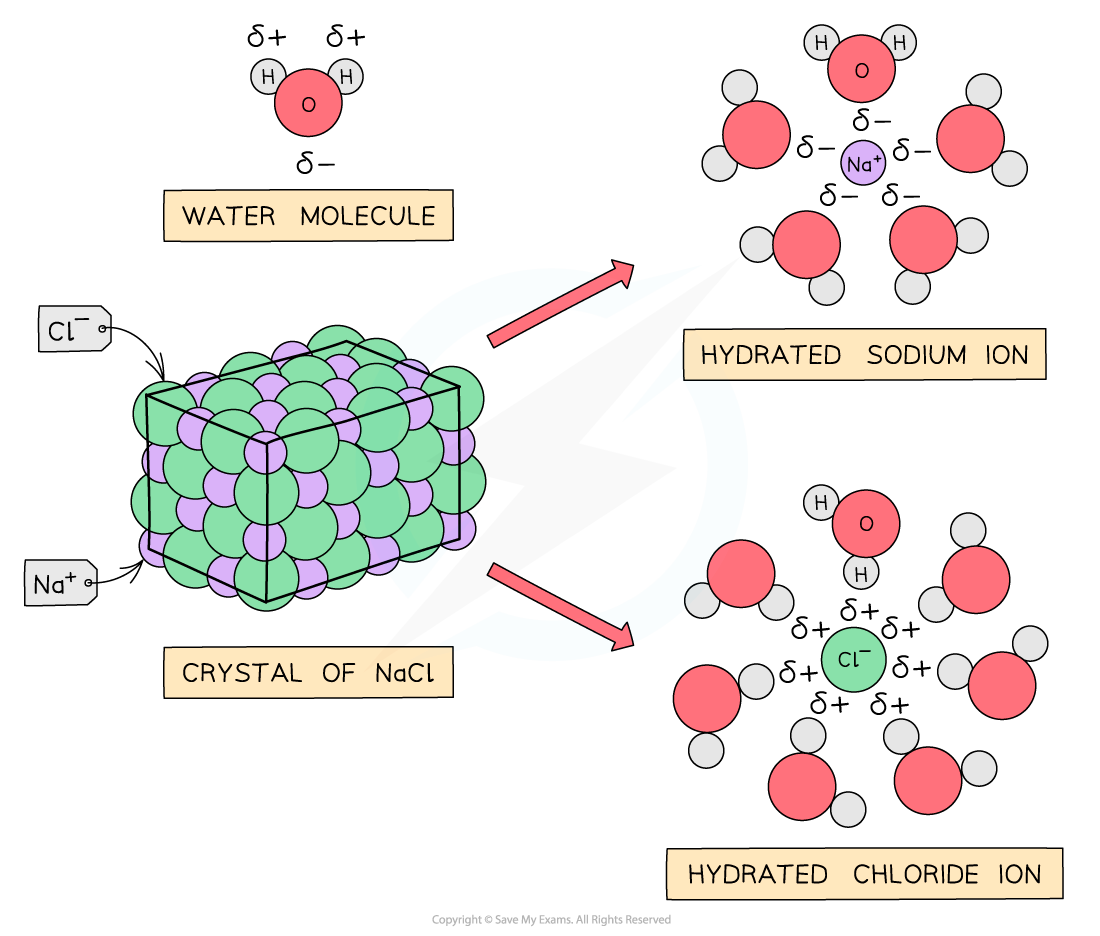
Are Ionic Compounds Poor Conductors Of Electricity . Ionic compounds have high melting points. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are hard and brittle. Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity.
from www.linstitute.net
Ionic compounds have high melting points. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic compounds are hard and brittle. Ionic solids are also poor conductors of electricity. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid.
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure
Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds have high melting points. Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic solids are also poor conductors of electricity. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid.
From slideplayer.com
Chapter 4 Formation of Compounds ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity. Ionic compounds are hard and brittle. Solutions of ionic. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
CHEMISTRY Matter and Change ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a. Ionic compounds have high melting points. Ionic solids are also poor conductors. Are Ionic Compounds Poor Conductors Of Electricity.
From www.numerade.com
SOLVED Chapter 1 A positively charged ion called a cation is formed Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic solids are also poor conductors of electricity. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Ionic Compounds & Metals ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity. Ionic compounds have high. Are Ionic Compounds Poor Conductors Of Electricity.
From www.shalom-education.com
Properties of Ionic Compounds GCSE Chemistry Revision Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Unit 6 Ionic and Covalent Bonding ppt download Are Ionic Compounds Poor Conductors Of Electricity Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are hard and brittle. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic compounds have high melting points.. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Flashcards for Ionic & Metallic Bonding ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds have high melting points. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are good conductors of. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Chapter 2 Atoms and Bonding ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Chemical BONDING. ppt download Are Ionic Compounds Poor Conductors Of Electricity Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state. Are Ionic Compounds Poor Conductors Of Electricity.
From www.toppr.com
Why don't ionic compounds have electrical conductivity as a solid but Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. The reason is—the strength of ionic bonds prevents ions from moving freely in. Are Ionic Compounds Poor Conductors Of Electricity.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.6 5 Ionic compounds Bonds, Structure Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic compounds do not conduct electricity in solid state because electron motion. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Ch. 8 Covalent Bonding College Chemistry. ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic. Are Ionic Compounds Poor Conductors Of Electricity.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 1.9.1 Explaining Conductivity翰林国际教育 Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic compounds have high melting points. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same. Are Ionic Compounds Poor Conductors Of Electricity.
From brainly.com
Write the empirical formula of at least four binary ionic compounds Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic compounds have high melting points. Ionic compounds do not conduct electricity in solid. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Chapter 5 Metals Vs. Nonmetals ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. The reason is—the strength of ionic bonds prevents ions. Are Ionic Compounds Poor Conductors Of Electricity.
From www.myxxgirl.com
Ionic Vs Covalent Which Is Which And How To Tell Them Apart My XXX Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds have high melting points. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are hard and brittle. Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that. Are Ionic Compounds Poor Conductors Of Electricity.
From askfilo.com
Covalent compounds are poor conductors of electricity. (ii) Carbon compo.. Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Bonding Ionic Bonding. ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity. Ionic compounds have high melting points. Ionic compounds do. Are Ionic Compounds Poor Conductors Of Electricity.
From h-o-m-e.org
Ionic Compounds' Electrical Conductivity Assessed Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic. Are Ionic Compounds Poor Conductors Of Electricity.
From philschatz.com
Electrolytes · Chemistry Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds have high melting points. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic compounds are hard and brittle. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic. Are Ionic Compounds Poor Conductors Of Electricity.
From exovzbqxo.blob.core.windows.net
Are Ionic Compounds Conductors Of Electricity at Paul Dejesus blog Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic compounds have high melting points. The reason is—the strength of ionic bonds prevents. Are Ionic Compounds Poor Conductors Of Electricity.
From www.pinterest.com
Properties of Ionic Compounds Ionic compound, Ionic, Compounds Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic solids are also poor conductors of electricity. Ionic compounds have high melting points. Ionic solids are also poor conductors of electricity. Are Ionic Compounds Poor Conductors Of Electricity.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Are Ionic Compounds Poor Conductors Of Electricity The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic compounds have high melting points. Ionic compounds are hard and brittle. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity. Solutions of ionic compounds and melted ionic. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
11/17 8th Grade Agenda Learning Objective Covalent Bonding ppt Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds have high melting points. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Chemical Bonding Chapter ppt download Are Ionic Compounds Poor Conductors Of Electricity Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds have high melting points. Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
7.2 Objectives Describe the formation of ionic bonds and the structure Are Ionic Compounds Poor Conductors Of Electricity Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds are hard and brittle. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Chapter 7 Ionic Bonding & the Formation of Ionic Compounds ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds are good conductors of electricity in the molten state or in solution as they have ions that can move and carry a. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic solids are also poor conductors of electricity for. Are Ionic Compounds Poor Conductors Of Electricity.
From www.teachoo.com
The table shown below gives information about four Chemistry MCQ Are Ionic Compounds Poor Conductors Of Electricity Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic compounds. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Ionic compounds tend to be strong electrolytes. ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic solids are also poor conductors of electricity. Ionic solids are also poor conductors of electricity for the same reason—the strength of. Are Ionic Compounds Poor Conductors Of Electricity.
From brainly.in
Write an activitu to show that ionic compounds are good conductors of Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic compounds have high melting points. Ionic compounds are hard and brittle. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Chem. warmup What is the difference between an ionic and covalent Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. Ionic solids are also poor conductors of electricity for the same reason—the strength of. Are Ionic Compounds Poor Conductors Of Electricity.
From askfilo.com
[1 mark](2) Covalent compounds are generally poor conductors of electric.. Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic solids are also poor conductors of electricity for. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Intramolecular Forces Intermolecular Forces ppt download Are Ionic Compounds Poor Conductors Of Electricity Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic compounds are. Are Ionic Compounds Poor Conductors Of Electricity.
From slideplayer.com
Chem. warmup What is the difference between an ionic and covalent Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds are hard and brittle. Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not. Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic solids are also poor conductors of electricity for the same reason—the. Are Ionic Compounds Poor Conductors Of Electricity.
From brainly.com
Write the empirical formula for at least four ionic compounds that Are Ionic Compounds Poor Conductors Of Electricity Ionic compounds do not conduct electricity in solid state because electron motion results in electricity being conducted but in solid state the constitutive particles are strongly bonded. Ionic compounds are hard and brittle. The reason is—the strength of ionic bonds prevents ions from moving freely in the. Ionic solids are also poor conductors of electricity for the same reason—the strength. Are Ionic Compounds Poor Conductors Of Electricity.