Lead Carbonate And Sulfuric Acid Balanced Equation . Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chemical equation (h2o = h + o) 🛠️ A net ionic equation is the most accurate representation of the actual chemical process that occurs. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation:
from www.thesciencehive.co.uk
Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Chemical equation (h2o = h + o) 🛠️ A net ionic equation is the most accurate representation of the actual chemical process that occurs.
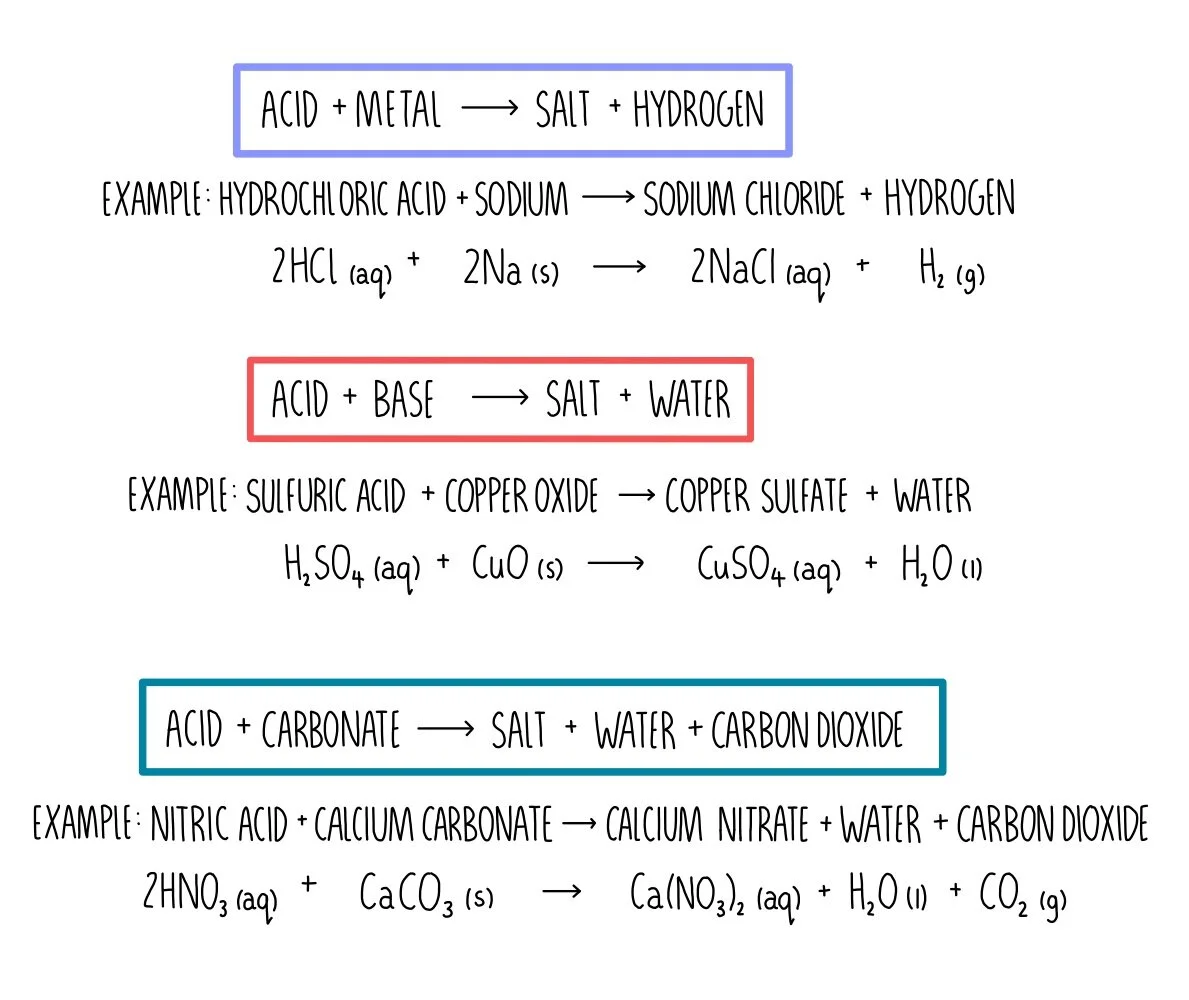
Acids, Bases and Salt Preparations (GCSE) — the science hive
Lead Carbonate And Sulfuric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. A net ionic equation is the most accurate representation of the actual chemical process that occurs. Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates.
From fyoawlxlr.blob.core.windows.net
Lead Carbonate And Sulfuric Acid Equation at Bonnie Siemens blog Lead Carbonate And Sulfuric Acid Balanced Equation Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: A net ionic equation is the most accurate representation of the actual chemical process that occurs. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to balance chemical equations using the law of conservation. Lead Carbonate And Sulfuric Acid Balanced Equation.
From diya-jolpblogcooper.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Lead Carbonate And Sulfuric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Use the calculator below to balance chemical equations and determine the type of reaction. Lead Carbonate And Sulfuric Acid Balanced Equation.
From tootsiebeuys.blogspot.com
Calcium Carbonate Sulphuric Acid Lead Carbonate And Sulfuric Acid Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. A net ionic equation is the most accurate representation of the actual chemical process that occurs. Enter an ionic equation and get its complete and net ionic equations,. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.slideshare.net
Chemical Reactions Lead Carbonate And Sulfuric Acid Balanced Equation Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Chemical equation (h2o = h + o) 🛠️ Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.youtube.com
How to Write the Net Ionic Equation for Fe(OH)3 + HNO3 = Fe(NO3)3 + H2O YouTube Lead Carbonate And Sulfuric Acid Balanced Equation A net ionic equation is the most accurate representation of the actual chemical process that occurs. Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to balance chemical equations using the law of conservation of. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED write the balanced molecular equation for sulfuric acid and sodium carbonate Lead Carbonate And Sulfuric Acid Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Chemical equation (h2o = h + o) 🛠️ Learn how to derive, write and balance chemical equations in molecular,. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.slideshare.net
Reaction types Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: A net ionic equation is the most accurate representation of the actual chemical process that occurs. Learn how to derive, write and. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.youtube.com
Write the balanced ionic equation for the reaction of sodium bicarbonate with sulphuric acid Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Chemical equation (h2o = h + o) 🛠️ Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equation for the reaction of sodium carbonate and sulfuric acid. How Lead Carbonate And Sulfuric Acid Balanced Equation Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. A net. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Write balanced chemical equations. 2) Aqueous lithium hydroxide neutralizes sulfuric Lead Carbonate And Sulfuric Acid Balanced Equation Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Chemical equation (h2o = h + o) 🛠️ Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: A net ionic equation is the most accurate representation of the actual chemical process that occurs.. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.toppr.com
Convert the following word equations into molecular equation and balance them. 1) Ammonium Lead Carbonate And Sulfuric Acid Balanced Equation A net ionic equation is the most accurate representation of the actual chemical process that occurs. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Chemical equation (h2o = h + o) 🛠️. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Lead Carbonate And Sulfuric Acid Balanced Equation Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to balance chemical equations using the law of. Lead Carbonate And Sulfuric Acid Balanced Equation.
From meryes.weebly.com
Balanced chemical equation with state symbols calculator meryes Lead Carbonate And Sulfuric Acid Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Chemical equation (h2o = h + o) 🛠️ Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: A net ionic equation is the most accurate representation of the. Lead Carbonate And Sulfuric Acid Balanced Equation.
From fyoawlxlr.blob.core.windows.net
Lead Carbonate And Sulfuric Acid Equation at Bonnie Siemens blog Lead Carbonate And Sulfuric Acid Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.slideshare.net
Chemical Reactions Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: A net ionic equation is the most accurate representation of the actual chemical process that occurs. Enter an ionic equation and get its complete and. Lead Carbonate And Sulfuric Acid Balanced Equation.
From treatybottle13.pythonanywhere.com
Ace Ammonia And Sulphuric Acid Balanced Equation Definition Of Double Displacement Reaction Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Aqueous solutions of sodium bicarbonate. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVEDWrite a balanced molecular equation and a net ionic equation for the following reactions Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Use the calculator below to. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVEDOn the basis of the general solubility rules given in Table 7.1, write a balanced Lead Carbonate And Sulfuric Acid Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Aqueous solutions of sodium bicarbonate and sulfuric acid react. Lead Carbonate And Sulfuric Acid Balanced Equation.
From aptaste.weebly.com
Sulfuric acid formula balance chemical equations calculator aptaste Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. A net ionic equation is the most accurate representation of the actual chemical process that occurs. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Use. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED A. Write balanced chemical equations Mercury (II) chloride + aluminum acetate Zinc Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. The chemical equation described in section 4.1 is. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVEDWrite complete ionic and net ionic equations for the reaction between sulfuric acid (H2 Lead Carbonate And Sulfuric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats.. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.dynamicscience.com.au
Chemistryacid reactionsionic equations Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction. Lead Carbonate And Sulfuric Acid Balanced Equation.
From exouyldhc.blob.core.windows.net
Lead Carbonate + Sulphuric Acid at Harold Petersen blog Lead Carbonate And Sulfuric Acid Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Chemical equation (h2o = h + o) 🛠️ Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. A net ionic equation is the most accurate representation of the actual chemical process that occurs. Learn how to. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.thesciencehive.co.uk
Acids, Bases and Salt Preparations (GCSE) — the science hive Lead Carbonate And Sulfuric Acid Balanced Equation Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. The chemical equation described in section 4.1. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED 5 . Predict the products of the following reactions and balance the chemical equations Lead Carbonate And Sulfuric Acid Balanced Equation The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Chemical equation (h2o = h + o) 🛠️ Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. Learn how to derive, write and balance chemical equations in molecular,. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon Lead Carbonate And Sulfuric Acid Balanced Equation Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: A net ionic equation is the most accurate representation of the actual chemical process that occurs. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. The chemical equation described in section 4.1 is. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED Write the balanced equations for the reactions of sulfuric acid with copper(Ihydroxide Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error.. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVEDCar Battery Car batteries use lead, lead(IV) oxide, and a sulfuric acid solution to Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Use the calculator below to balance chemical equations and determine the type of reaction (instructions). A net ionic equation is the most accurate representation of the actual chemical process that occurs. Enter an ionic equation and get its complete and net ionic equations,. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.numerade.com
SOLVED A 15.0 g sample of sodium carbonate reacts with excess sulfuric acid to form sodium Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. Chemical equation (h2o = h + o) 🛠️ Use the calculator below to balance chemical equations and determine the type of reaction (instructions). A net ionic equation is the most accurate representation of the actual chemical process that occurs. Enter an ionic equation. Lead Carbonate And Sulfuric Acid Balanced Equation.
From ava-chapter.blogspot.com
Copper Carbonate Sulphuric Acid Balanced Equation 81+ Pages Explanation [800kb] Updated Ava Lead Carbonate And Sulfuric Acid Balanced Equation Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: Learn how to derive, write and balance chemical equations in molecular, total ionic and net ionic formats. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Enter an. Lead Carbonate And Sulfuric Acid Balanced Equation.
From www.chegg.com
Solved Write a balanced chemical equation (including phases) Lead Carbonate And Sulfuric Acid Balanced Equation A net ionic equation is the most accurate representation of the actual chemical process that occurs. Aqueous solutions of sodium bicarbonate and sulfuric acid react to produce carbon dioxide according to the following equation: The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Use the calculator. Lead Carbonate And Sulfuric Acid Balanced Equation.
From fyoawlxlr.blob.core.windows.net
Lead Carbonate And Sulfuric Acid Equation at Bonnie Siemens blog Lead Carbonate And Sulfuric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Learn how to derive, write and balance chemical equations. Lead Carbonate And Sulfuric Acid Balanced Equation.
From aliananewsgaines.blogspot.com
What Is Formed When Calcium Carbonate Reacts With Sulfuric Acid Lead Carbonate And Sulfuric Acid Balanced Equation Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. The chemical equation described in section 4.1 is balanced, meaning that equal numbers of atoms for each element involved in the reaction are. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and. Lead Carbonate And Sulfuric Acid Balanced Equation.
From slideplayer.com
Barium Hydroxide Sulfuric Acid Reaction ppt download Lead Carbonate And Sulfuric Acid Balanced Equation Use the calculator below to balance chemical equations and determine the type of reaction (instructions). A net ionic equation is the most accurate representation of the actual chemical process that occurs. Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Aqueous. Lead Carbonate And Sulfuric Acid Balanced Equation.
From gioobnpqg.blob.core.windows.net
Lead Carbonate Balanced Equation at Lyle Hill blog Lead Carbonate And Sulfuric Acid Balanced Equation Chemical equation (h2o = h + o) 🛠️ Learn how to balance chemical equations using the law of conservation of mass and the method of trial and error. Enter an ionic equation and get its complete and net ionic equations, solubility states, spectator ions and precipitates. A net ionic equation is the most accurate representation of the actual chemical process. Lead Carbonate And Sulfuric Acid Balanced Equation.