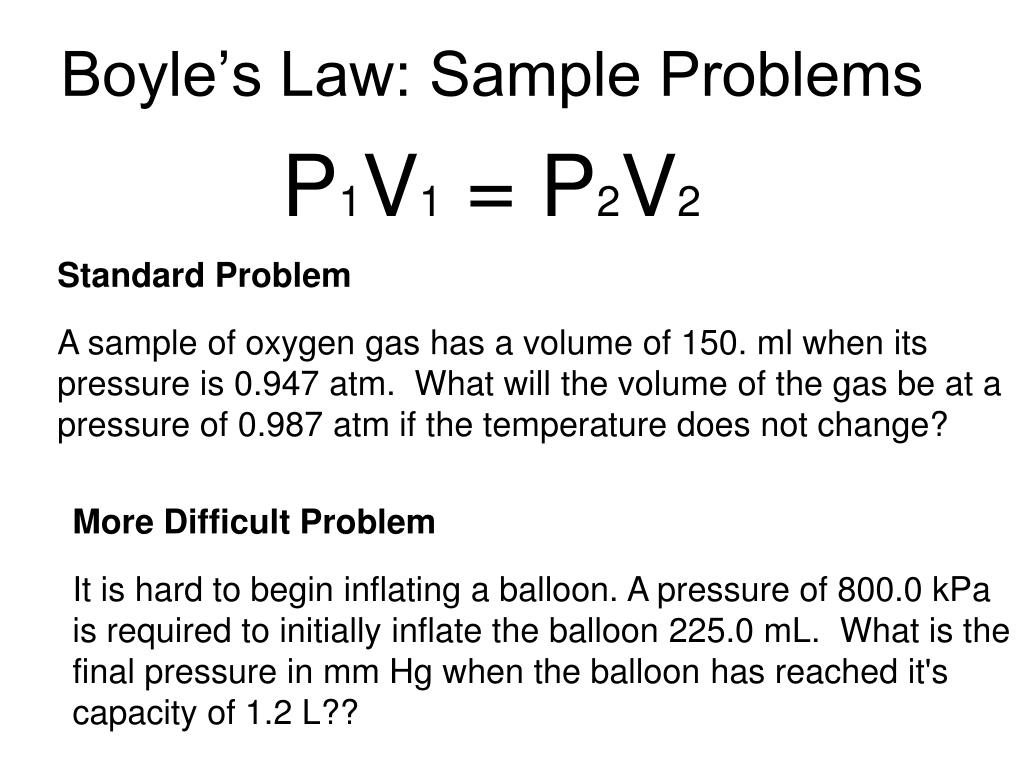
What Does Volume Mean In Gas . The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The particles gain kinetic energy and move faster. And, of course, you could redo this calculation to find the volume of 1 mole of an. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. They collide more often with greater force on. When the gas is heated, its volume increases.
from www.slideserve.com
And, of course, you could redo this calculation to find the volume of 1 mole of an. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The particles gain kinetic energy and move faster. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. When the gas is heated, its volume increases. They collide more often with greater force on.
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint
What Does Volume Mean In Gas When the gas is heated, its volume increases. When the gas is heated, its volume increases. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. They collide more often with greater force on. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. And, of course, you could redo this calculation to find the volume of 1 mole of an. The particles gain kinetic energy and move faster.
From study.com
Matter, Mass & Volume Differences & Examples Lesson What Does Volume Mean In Gas And, of course, you could redo this calculation to find the volume of 1 mole of an. They collide more often with greater force on. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature. What Does Volume Mean In Gas.
From socratic.org
A sample of a gas has a volume of 2.0 liters at a pressure of 1.0 What Does Volume Mean In Gas And, of course, you could redo this calculation to find the volume of 1 mole of an. They collide more often with greater force on. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. When the gas is heated, its volume increases. The volume of a gas is inversely proportional to. What Does Volume Mean In Gas.
From www.slideserve.com
PPT 1. The volume of a gas at 99.0 kPa is 300.0 mL. If the pressure What Does Volume Mean In Gas The particles gain kinetic energy and move faster. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. They collide more often with greater force on. The molar volume of an ideal gas is therefore 22.4 dm. What Does Volume Mean In Gas.
From www.britannica.com
Ideal gas Definition, Equation, Properties, & Facts Britannica What Does Volume Mean In Gas Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. The particles gain kinetic energy and move faster. And, of course, you could redo this calculation to find the volume of 1 mole of an. Where p is. What Does Volume Mean In Gas.
From sciencenotes.org
Specific Volume Definition and Examples What Does Volume Mean In Gas They collide more often with greater force on. And, of course, you could redo this calculation to find the volume of 1 mole of an. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Volume of a gas is directly proportional to the amount of gas at. What Does Volume Mean In Gas.
From www.slideserve.com
PPT Pressure, Volume, Temperature The Gas Laws PowerPoint What Does Volume Mean In Gas Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. The particles gain kinetic energy and move faster. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the. What Does Volume Mean In Gas.
From sciencenotes.org
Real Gas vs Ideal Gas What Does Volume Mean In Gas Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Where p is the pressure of a gas, v is its volume, n is the number of moles of the. What Does Volume Mean In Gas.
From www.youtube.com
How To Calculate Gas Volumes Chemical Calculations Chemistry What Does Volume Mean In Gas They collide more often with greater force on. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. The particles gain kinetic energy and move faster. Volume of a gas is directly proportional to the amount of. What Does Volume Mean In Gas.
From www.dreamstime.com
Boyle S Law Showing the Pressure and Volume Relationship Stock Vector What Does Volume Mean In Gas And, of course, you could redo this calculation to find the volume of 1 mole of an. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. They collide more often with greater force on. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Where p is. What Does Volume Mean In Gas.
From www.worksheetsplanet.com
What is Volume Meaning & Definition What Does Volume Mean In Gas And, of course, you could redo this calculation to find the volume of 1 mole of an. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature. What Does Volume Mean In Gas.
From www.youtube.com
Properties of gases class 9 how to show that gases do not have a What Does Volume Mean In Gas They collide more often with greater force on. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. The particles gain kinetic energy. What Does Volume Mean In Gas.
From users.highland.edu
Molar Volume of a Gas What Does Volume Mean In Gas The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The particles gain kinetic energy and move faster. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and. What Does Volume Mean In Gas.
From masterconceptsinchemistry.com
What’s the relationship between pressure and volume of gas? Core What Does Volume Mean In Gas The particles gain kinetic energy and move faster. When the gas is heated, its volume increases. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. They collide more often with greater force on. Volume of a gas is directly proportional to the amount of gas at a. What Does Volume Mean In Gas.
From www.grc.nasa.gov
Specific Volume What Does Volume Mean In Gas The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. They collide more often with greater force on. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The particles gain kinetic energy and move faster. The volume of a gas is inversely proportional to its pressure and. What Does Volume Mean In Gas.
From dmgmwenda.blogspot.com
Volumen En Los Gases wenda What Does Volume Mean In Gas The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. They collide more often with. What Does Volume Mean In Gas.
From www.nagwa.com
Question Video Calculating Gas Volume after It Is Heated at Constant What Does Volume Mean In Gas Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The particles gain kinetic energy and move faster. When the gas is heated, its volume increases. They collide more often. What Does Volume Mean In Gas.
From mmerevise.co.uk
The Ideal Gas Equation MME What Does Volume Mean In Gas Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. When the gas is heated, its volume increases. They collide more often with greater force on. The volume of a gas is inversely proportional to its pressure. What Does Volume Mean In Gas.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal What Does Volume Mean In Gas The particles gain kinetic energy and move faster. When the gas is heated, its volume increases. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. And, of course, you could redo this calculation to find the. What Does Volume Mean In Gas.
From www.cuemath.com
Volume Formula Definition What is Volume Cuemath What Does Volume Mean In Gas And, of course, you could redo this calculation to find the volume of 1 mole of an. They collide more often with greater force on. When the gas is heated, its volume increases. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The molar volume of an. What Does Volume Mean In Gas.
From sciencenotes.org
Volume Definition in Science What Does Volume Mean In Gas The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. They collide more often with greater force on. And, of course, you could redo this calculation to find the volume. What Does Volume Mean In Gas.
From www.slideserve.com
PPT Gas Volumes L.O. PowerPoint Presentation, free download ID5877069 What Does Volume Mean In Gas The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. They collide more often with greater force on. The particles gain kinetic energy and move faster. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Volume of a gas is directly proportional to. What Does Volume Mean In Gas.
From www.youtube.com
An ideal gas is compressed to half its initial volume by means of What Does Volume Mean In Gas The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. They collide more often with greater force on. When the gas is heated, its volume increases. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The particles gain kinetic energy and move faster.. What Does Volume Mean In Gas.
From www.grc.nasa.gov
Gas Density What Does Volume Mean In Gas And, of course, you could redo this calculation to find the volume of 1 mole of an. They collide more often with greater force on. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. When the gas is heated, its volume increases. Where p is the pressure. What Does Volume Mean In Gas.
From www.youtube.com
Volume of 1 mole of any gas at STP using the ideal gas equation YouTube What Does Volume Mean In Gas The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. They collide more often with greater force on. When the gas is heated, its volume increases. The particles gain kinetic energy and move faster. Volume of a gas is directly proportional to the amount of gas at a. What Does Volume Mean In Gas.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law What Does Volume Mean In Gas Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. When the gas is heated, its volume increases. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The particles gain kinetic energy and move faster. And, of course, you. What Does Volume Mean In Gas.
From classnotes.gidemy.com
States of Matter Gidemy Class Notes What Does Volume Mean In Gas Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. They collide more often with greater force on. When the gas is heated, its volume increases. The particles gain kinetic energy and move faster. And, of course, you could redo this calculation to find the volume of 1 mole of an. The. What Does Volume Mean In Gas.
From www.slideserve.com
PPT Chapter 14 Gas Laws PowerPoint Presentation, free download ID What Does Volume Mean In Gas The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. And, of course, you could redo this calculation to find the volume of 1 mole of an. They collide more often with greater force. What Does Volume Mean In Gas.
From www.shalom-education.com
Volume and Pressure in Gases GCSE Physics Revision What Does Volume Mean In Gas They collide more often with greater force on. And, of course, you could redo this calculation to find the volume of 1 mole of an. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Where p is the pressure of a gas, v is its volume, n is the number of. What Does Volume Mean In Gas.
From sciencenotes.org
Ideal Gas Law Formula and Examples What Does Volume Mean In Gas The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The particles gain kinetic energy and move faster. And, of course, you could redo this calculation to find the volume. What Does Volume Mean In Gas.
From en.ppt-online.org
The ideal gas equation online presentation What Does Volume Mean In Gas Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. The particles gain kinetic energy and move faster. When. What Does Volume Mean In Gas.
From www.youtube.com
Gas volumes (AQA GCSE Chemistry) YouTube What Does Volume Mean In Gas They collide more often with greater force on. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. When the gas is heated, its volume increases. Where p is the pressure of a gas,. What Does Volume Mean In Gas.
From www.youtube.com
1.4.6 Solve problems involving temperature, pressure and volume for an What Does Volume Mean In Gas Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. The molar volume of an ideal gas is therefore 22.4 dm 3 at stp. The particles gain kinetic energy and. What Does Volume Mean In Gas.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock What Does Volume Mean In Gas The particles gain kinetic energy and move faster. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. They collide more often with greater force on. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on. What Does Volume Mean In Gas.
From www.slideserve.com
PPT Gas Volumes L.O. PowerPoint Presentation, free download ID5877069 What Does Volume Mean In Gas When the gas is heated, its volume increases. Volume of a gas is directly proportional to the amount of gas at a constant temperature and pressure. The volume of a gas is inversely proportional to its pressure and directly proportional to its temperature and the amount of gas. Where p is the pressure of a gas, v is its volume,. What Does Volume Mean In Gas.
From www.youtube.com
Constant Volume Heating of a Gas YouTube What Does Volume Mean In Gas When the gas is heated, its volume increases. Where p is the pressure of a gas, v is its volume, n is the number of moles of the gas, t is its temperature on the kelvin scale, and r is a. And, of course, you could redo this calculation to find the volume of 1 mole of an. The particles. What Does Volume Mean In Gas.