Calorimetry Experiment Lab Report Discussion . Chemical reactions involve the release or consumption of energy, usually in the. To apply these δh values in a hess’s law calculation to. Lab session 9, experiment 8: Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Flowing from an object at a higher temperature to one at a lower temperature.
from www.studocu.com
The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Lab session 9, experiment 8: Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Chemical reactions involve the release or consumption of energy, usually in the. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. To apply these δh values in a hess’s law calculation to. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. Flowing from an object at a higher temperature to one at a lower temperature.
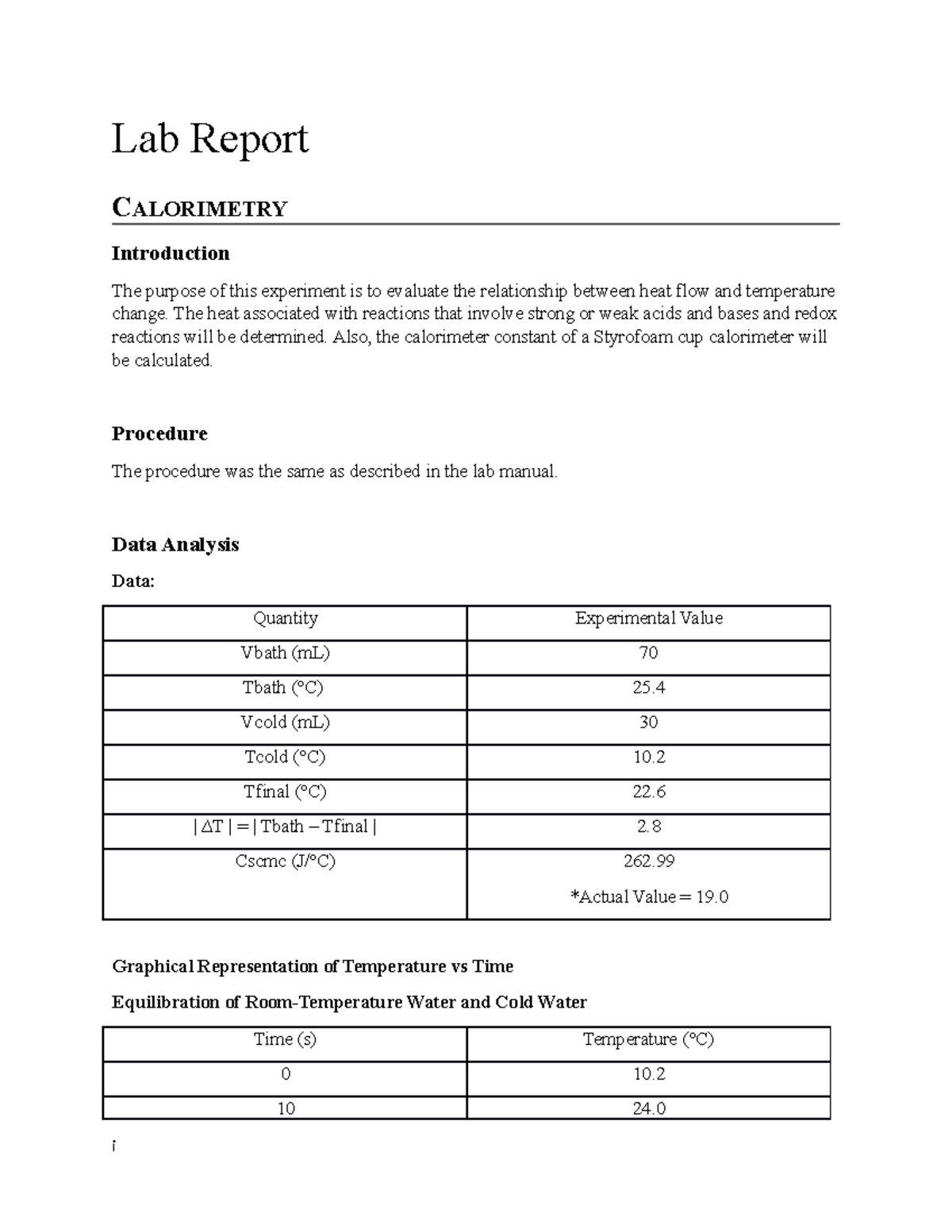
Lab Report Calorimetry Lab Report CALORIMETRY Introduction The
Calorimetry Experiment Lab Report Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Chemical reactions involve the release or consumption of energy, usually in the. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. To apply these δh values in a hess’s law calculation to. Flowing from an object at a higher temperature to one at a lower temperature. Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Experiment Lab Report Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. Lab session 9, experiment 8: The discussion is the most important part of. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Name Mahma Ahmed Partner Wala Naeem Student Calorimetry Experiment Lab Report Discussion To apply these δh values in a hess’s law calculation to. Lab session 9, experiment 8: To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Specific heat is an intensive property of a. Calorimetry Experiment Lab Report Discussion.
From www.docsity.com
Calorimetry lab report Study Guides, Projects, Research Chemistry Calorimetry Experiment Lab Report Discussion To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Chemical reactions involve the release or consumption. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Physics 212 General Physics With Calculus 212 Calorimetry Experiment Lab Report Discussion Lab session 9, experiment 8: To apply these δh values in a hess’s law calculation to. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Chemical reactions involve the. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Lab Report Calorimetry Lab Report CALORIMETRY Introduction The Calorimetry Experiment Lab Report Discussion Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Flowing from an object at a higher temperature to one at a lower temperature. The discussion is the most important part. Calorimetry Experiment Lab Report Discussion.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Calorimetry Experiment Lab Report Discussion To apply these δh values in a hess’s law calculation to. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Lab session 9, experiment 8: To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Experiment 6 ∙ calorimetry 6‐2 experiment. Calorimetry Experiment Lab Report Discussion.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Lab Report Discussion To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Experiment 6 ∙ calorimetry 6‐2. Calorimetry Experiment Lab Report Discussion.
From www.chegg.com
Solved Calorimetry Experiment Lab Report Worksheet Name Calorimetry Experiment Lab Report Discussion Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. In this experiment you. Calorimetry Experiment Lab Report Discussion.
From www.studypool.com
SOLUTION CHEM 162 Calorimetry and Hess’s Law Lab Report Studypool Calorimetry Experiment Lab Report Discussion To apply these δh values in a hess’s law calculation to. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Flowing from an object at a higher temperature to one at a lower temperature. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the. Calorimetry Experiment Lab Report Discussion.
From www.scribd.com
Calorimetry Lab SE Calorie Heat Capacity Calorimetry Experiment Lab Report Discussion Flowing from an object at a higher temperature to one at a lower temperature. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. To experimentally measure the δh values of two. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Experiment Lab Report Discussion To apply these δh values in a hess’s law calculation to. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. Chemical reactions involve the release or consumption of energy, usually in the. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only.. Calorimetry Experiment Lab Report Discussion.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry Lab Sec Name Calorimetry Experiment Lab Report Discussion The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. In this. Calorimetry Experiment Lab Report Discussion.
From www.thinkswap.com
Calorimetry Lab Report PHYS1006 Foundations of Physics Curtin Calorimetry Experiment Lab Report Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. To apply these δh values in a hess’s law calculation to. Flowing from an object at a higher temperature to one. Calorimetry Experiment Lab Report Discussion.
From www.scribd.com
Calorimetry Experiment Lab Report PDF Sodium Hydroxide Calorimetry Experiment Lab Report Discussion Lab session 9, experiment 8: The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Flowing from an object at a higher temperature to one at a lower temperature. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. In this experiment you. Calorimetry Experiment Lab Report Discussion.
From www.chegg.com
Solved Experiment 25 Report Sheet Calorimetry / 613 Lob Sec. Calorimetry Experiment Lab Report Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Chemical reactions involve the release or consumption of energy, usually in the. Lab session 9, experiment 8: The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Specific. Calorimetry Experiment Lab Report Discussion.
From www.youtube.com
How to calculate analysis results L3 Lab Report Calorimetry YouTube Calorimetry Experiment Lab Report Discussion Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. To apply these δh values in a hess’s law calculation to. Flowing from an object at a higher. Calorimetry Experiment Lab Report Discussion.
From studylib.net
Student instructions and lab report Calorimetry Experiment Lab Report Discussion Lab session 9, experiment 8: Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy.. Calorimetry Experiment Lab Report Discussion.
From www.pdfprof.com
calorimetry experiment lab report conclusion Calorimetry Experiment Lab Report Discussion To apply these δh values in a hess’s law calculation to. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. Abstract the main purpose of the calorimetry experiment is to measure. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Coffeecup Calorimetry Experiment Formal Lab Report I. Introduction Calorimetry Experiment Lab Report Discussion To apply these δh values in a hess’s law calculation to. Lab session 9, experiment 8: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Flowing from an object at a higher temperature to one at a lower temperature. To experimentally measure the δh values. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Lab report. Experiment 6. (1 week) (LCA Calorimetry Experiment Lab Report Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. Chemical reactions involve the release or consumption of energy, usually in the. The. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
P calorimetry 25 lab report StuDocu Calorimetry Experiment Lab Report Discussion The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. To apply these δh values in a hess’s law calculation to. Abstract the main purpose of the calorimetry. Calorimetry Experiment Lab Report Discussion.
From pdfprof.com
experiment 25 calorimetry lab report Calorimetry Experiment Lab Report Discussion Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. Chemical reactions involve the release or consumption of energy, usually in the. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Abstract the main purpose of the calorimetry experiment is to measure. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Write an abstract for the calorimetry Calorimetry Experiment Lab Report Discussion Lab session 9, experiment 8: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry.. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry pt. 1 lab report CALORIMETRY PART 1 S PECIFIC HEAT C Calorimetry Experiment Lab Report Discussion Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. Flowing from an object at a higher temperature to one at a lower temperature. Chemical. Calorimetry Experiment Lab Report Discussion.
From www.studypool.com
SOLUTION Calorimetry Enthalpy of Reaction and Heat Capacity of a Calorimetry Experiment Lab Report Discussion Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. Chemical reactions involve the release or consumption of energy, usually in the. Lab session 9, experiment 8: To experimentally measure the δh values of two. Calorimetry Experiment Lab Report Discussion.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template Calorimetry Experiment Lab Report Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the. Calorimetry Experiment Lab Report Discussion.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimetry Experiment Lab Report Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Chemical reactions involve the release or consumption of energy, usually in the. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. To apply these δh values in a hess’s law. Calorimetry Experiment Lab Report Discussion.
From www.studypool.com
SOLUTION Student exploration calorimetry lab Studypool Calorimetry Experiment Lab Report Discussion The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Calorimetry is the science of measuring heat flow, and heat is defined as thermal. Calorimetry Experiment Lab Report Discussion.
From studylib.net
06.03 Calorimetry Lab Report Calorimetry Experiment Lab Report Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry mathematical development the calorimeter constant ccal calorimetry is the science of measuring the quantities of. Chemical reactions involve the release or consumption of energy, usually in the. Flowing from an object at. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry Lab Report Experiment 6. Calorimetry Purpose The purpose Calorimetry Experiment Lab Report Discussion Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
CoffeeCup Calorimetry Experiment Lab Report with answers and solutions Calorimetry Experiment Lab Report Discussion Lab session 9, experiment 8: Chemical reactions involve the release or consumption of energy, usually in the. The discussion is the most important part of the laboratory report, because it is here that you demonstrate that you not only. To apply these δh values in a hess’s law calculation to. Flowing from an object at a higher temperature to one. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Formal Lab Report Calorimetry Experiment 25 CALORIMETRY Abstract Calorimetry Experiment Lab Report Discussion Specific heat is an intensive property of a single phase (solid, liquid or gas) sample that describes how the temperature. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. To apply these δh values in a hess’s law calculation to. Chemical reactions involve the release or consumption of energy, usually in the. Calorimetry. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Calorimetry (Experiment 3) Laboratory Report Calorimetry (Experiment Calorimetry Experiment Lab Report Discussion Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. To apply these δh values in a hess’s law calculation to. Calorimetry is the science of measuring heat flow, and heat is defined as thermal energy. In this experiment you will heat a known mass of a metal to a. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Experiment 25 Calorimetry Laboratory Report2 Experiment 25 Calorimetry Experiment Lab Report Discussion To apply these δh values in a hess’s law calculation to. Flowing from an object at a higher temperature to one at a lower temperature. Abstract the main purpose of the calorimetry experiment is to measure the enthalpy, specific heat capacity, of substances using a. Specific heat is an intensive property of a single phase (solid, liquid or gas) sample. Calorimetry Experiment Lab Report Discussion.
From www.studocu.com
Experiment 25 Calorimetry Prelaboratory Assignment CHEM 005 Studocu Calorimetry Experiment Lab Report Discussion In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that. Chemical reactions involve the release or consumption of energy, usually in the. To experimentally measure the δh values of two reactions using the technique of constant pressure calorimetry. Experiment 6 ∙ calorimetry 6‐2 experiment 6 calorimetry. Calorimetry Experiment Lab Report Discussion.