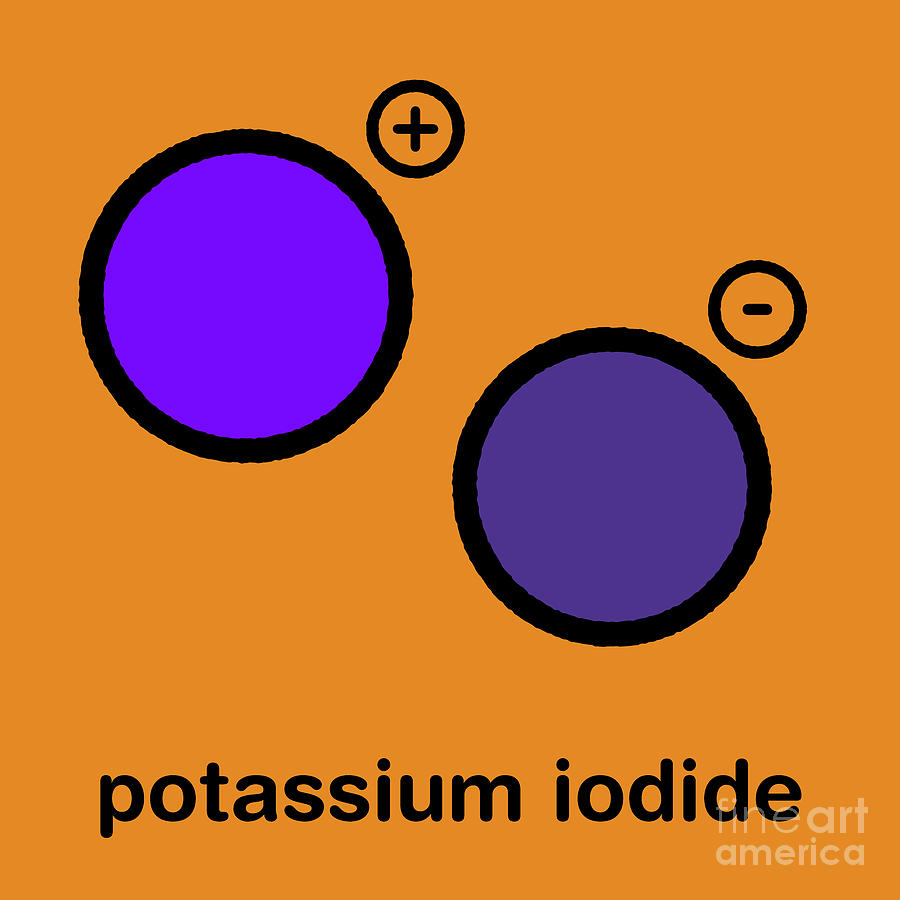
Potassium Iodide Valence Electrons . The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. It is equivalent to zero. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. 119 rows valence electrons: The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The formula for potassium iodide is ki k i.
from mavink.com
Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The formula for potassium iodide is ki k i. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). It is equivalent to zero. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. 119 rows valence electrons: The total formal charge of this combination, composed of a metal and a nonmetal, is neutral;
Potassium Iodide Structure
Potassium Iodide Valence Electrons Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 119 rows valence electrons: The attractive interactions between its component ions result in the ionic compound potassium iodide formation. The formula for potassium iodide is ki k i. It is equivalent to zero. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.
From stock.adobe.com
Vecteur Stock Potassium Iodide KI molecule. Simple molecular formula Potassium Iodide Valence Electrons 119 rows valence electrons: In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond. Potassium Iodide Valence Electrons.
From exatin.info
Iodine Dot Diagram exatin.info Potassium Iodide Valence Electrons Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. It is equivalent to zero. The formula for potassium iodide is ki k i. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. 119 rows valence electrons: 93. Potassium Iodide Valence Electrons.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure Potassium Iodide Valence Electrons The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; 119 rows valence electrons: Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. In ki, iodine (i) is. Potassium Iodide Valence Electrons.
From topblogtenz.com
Potassium Orbital diagram, Electron configuration, and Valence electrons Potassium Iodide Valence Electrons Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). The formula for potassium iodide is ki k. Potassium Iodide Valence Electrons.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure Potassium Iodide Valence Electrons The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 119 rows valence electrons: 93 rows you may. Potassium Iodide Valence Electrons.
From favpng.com
Lewis Structure Potassium Iodide Potassium Oxide Electron, PNG Potassium Iodide Valence Electrons Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). The formula for potassium iodide is ki k i. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an. Potassium Iodide Valence Electrons.
From mavink.com
Potassium Iodide Structure Potassium Iodide Valence Electrons It is equivalent to zero. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 119 rows valence electrons: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In ki, iodine (i) is the central. Potassium Iodide Valence Electrons.
From truyenhinhcapsongthu.net
How Many Protons, Neutrons And Electrons Does Potassium Have? Potassium Iodide Valence Electrons It is equivalent to zero. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The formula for potassium iodide is ki k i. The total formal charge of this combination, composed of a metal. Potassium Iodide Valence Electrons.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure Potassium Iodide Valence Electrons The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). The attractive interactions between its component ions result in the ionic compound potassium iodide formation. Either atoms gain enough electrons to have eight. Potassium Iodide Valence Electrons.
From proper-cooking.info
Potassium Iodide Lewis Dot Structure Potassium Iodide Valence Electrons It is equivalent to zero. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. 119 rows valence electrons: The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; In ki, iodine (i) is the central atom since it is less electronegative than. Potassium Iodide Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Potassium (K)? Potassium Iodide Valence Electrons In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). 119 rows valence electrons: The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; The formula for potassium iodide is ki k i. The attractive interactions between its component ions result in the ionic compound potassium iodide formation.. Potassium Iodide Valence Electrons.
From www.shutterstock.com
Potassium Iodide Properties Chemical Compound Structure Stock Vector Potassium Iodide Valence Electrons The attractive interactions between its component ions result in the ionic compound potassium iodide formation. The formula for potassium iodide is ki k i. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. In ki, iodine (i) is the central atom since it is less electronegative than potassium. Potassium Iodide Valence Electrons.
From ar.inspiredpencil.com
Potassium Valence Electron Number Potassium Iodide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. It is equivalent to zero. The lewis structure of potassium iodide, ki, consists of one potassium. Potassium Iodide Valence Electrons.
From www.clipartkey.com
Iodine Dot Cross Diagram Electron Potassium Iodide Shell Pattern Of Potassium Iodide Valence Electrons The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that.. Potassium Iodide Valence Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Potassium (K) Have? Potassium Iodide Valence Electrons The attractive interactions between its component ions result in the ionic compound potassium iodide formation. The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺). Potassium Iodide Valence Electrons.
From www.slideshare.net
Bonding Basics Potassium Iodide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. The formula for potassium iodide is ki k i. The attractive interactions between its component. Potassium Iodide Valence Electrons.
From www.shutterstock.com
Potassium Iodide Ki Molecule Simple Molecular vetor stock (livre de Potassium Iodide Valence Electrons It is equivalent to zero. 119 rows valence electrons: The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). The attractive interactions between its component ions result in the ionic compound potassium iodide. Potassium Iodide Valence Electrons.
From periodictable.me
Potassium Electron Configuration (K) with Orbital Diagram Potassium Iodide Valence Electrons 119 rows valence electrons: The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. The formula for potassium iodide is ki k i. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). The attractive interactions between its component ions result in the. Potassium Iodide Valence Electrons.
From www.sciencecoverage.com
Valency of Potassium How many valence electrons does Potassium (K) have? Potassium Iodide Valence Electrons The formula for potassium iodide is ki k i. 119 rows valence electrons: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; The attractive interactions between its component ions. Potassium Iodide Valence Electrons.
From www.alamy.com
Potassium iodide chemical structure, illustration Stock Photo Alamy Potassium Iodide Valence Electrons The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. It is equivalent to zero. 119 rows valence electrons: The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an. Potassium Iodide Valence Electrons.
From www.sciencephoto.com
Potassium iodide chemical structure, illustration Stock Image F028 Potassium Iodide Valence Electrons The formula for potassium iodide is ki k i. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). 119 rows valence electrons: Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 93 rows you may assume the valences of the chemical elements—the. Potassium Iodide Valence Electrons.
From www.animalia-life.club
Potassium Atom Diagram Potassium Iodide Valence Electrons The attractive interactions between its component ions result in the ionic compound potassium iodide formation. It is equivalent to zero. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will. Potassium Iodide Valence Electrons.
From www.benjamin-mills.com
Electron configurations Potassium Iodide Valence Electrons The formula for potassium iodide is ki k i. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). 119 rows valence electrons: Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The attractive interactions between its component ions result in the ionic. Potassium Iodide Valence Electrons.
From chemistry291.blogspot.com
How Many Valence Electrons Does Potassium Have?number of valence Potassium Iodide Valence Electrons The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). 93 rows you may assume the valences of. Potassium Iodide Valence Electrons.
From ar.inspiredpencil.com
Potassium Iodide Structure Potassium Iodide Valence Electrons The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. 93 rows you. Potassium Iodide Valence Electrons.
From periodictable.me
How Do You Find The Electron Configuration for Potassium (K) Dynamic Potassium Iodide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It is equivalent to zero. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. In ki, iodine (i) is the central atom since it. Potassium Iodide Valence Electrons.
From www.chemistrylearner.com
Iodine Facts, Symbol, Discovery, Properties, Uses Potassium Iodide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. The attractive interactions between its component ions result in the ionic compound potassium iodide formation.. Potassium Iodide Valence Electrons.
From mavink.com
Potassium Iodide Chemical Structure Potassium Iodide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. It is equivalent to zero. The formula for potassium iodide is ki k i. The lewis structure of potassium iodide, ki,. Potassium Iodide Valence Electrons.
From newtondesk.com
Potassium K (Element 19) of Periodic Table Elements FlashCards Potassium Iodide Valence Electrons The attractive interactions between its component ions result in the ionic compound potassium iodide formation. The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. It is equivalent to zero. 93 rows you. Potassium Iodide Valence Electrons.
From www.kindpng.com
Valence Electrons Of Potassium, HD Png Download kindpng Potassium Iodide Valence Electrons In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It is equivalent to zero. Either atoms gain enough electrons to have eight electrons in the valence shell and become. Potassium Iodide Valence Electrons.
From mavink.com
Potassium Iodide Lewis Dot Structure Potassium Iodide Valence Electrons It is equivalent to zero. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. In ki, iodine (i) is the central atom since it is less electronegative than potassium (k). 119 rows valence electrons: The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; The formula for potassium. Potassium Iodide Valence Electrons.
From awesomehome.co
Potassium Periodic Table Protons Neutrons And Electrons Awesome Home Potassium Iodide Valence Electrons Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron to one iodide ion (i⁻),. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will. Potassium Iodide Valence Electrons.
From www.vrogue.co
Potassium Electron Configuration K With Orbital Diagr vrogue.co Potassium Iodide Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The lewis structure of potassium iodide, ki, consists of one potassium ion (k⁺) donating an electron. Potassium Iodide Valence Electrons.
From diagramweb.net
Lewis Dot Diagram Iodine Potassium Iodide Valence Electrons Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. The total formal charge of this combination, composed of a metal and a nonmetal, is neutral; 119 rows valence electrons: In ki, iodine (i) is. Potassium Iodide Valence Electrons.
From www.animalia-life.club
Potassium Iodide Lewis Dot Structure Potassium Iodide Valence Electrons The formula for potassium iodide is ki k i. The attractive interactions between its component ions result in the ionic compound potassium iodide formation. It is equivalent to zero. 119 rows valence electrons: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. In ki, iodine. Potassium Iodide Valence Electrons.