Zinc And Hydrochloric Acid Mass . The reaction between hydrochloric acid and zinc. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen causes bubbling during the. Properties of zinc and specifics of its interactions with hcl. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. 2hcl + zn → zncl 2 + h 2.
from www.chegg.com
2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The reaction between hydrochloric acid and zinc. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Hydrochloric acid + zinc → zinc chloride + hydrogen. Properties of zinc and specifics of its interactions with hcl. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description:
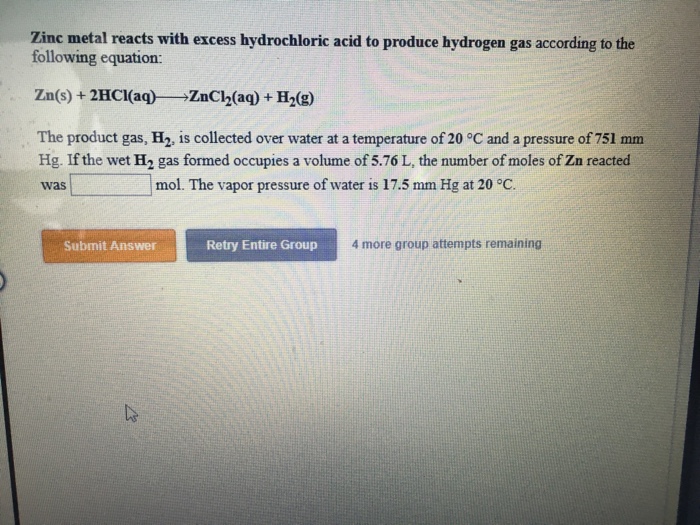
Solved Zinc metal reacts with excess hydrochloric acid to
Zinc And Hydrochloric Acid Mass Properties of zinc and specifics of its interactions with hcl. Properties of zinc and specifics of its interactions with hcl. Hydrochloric acid + zinc → zinc chloride + hydrogen. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. The reaction between hydrochloric acid and zinc. 2hcl + zn → zncl 2 + h 2. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The hydrogen causes bubbling during the. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction.
From www.chegg.com
Solved Zinc metal reacts with excess hydrochloric acid to Zinc And Hydrochloric Acid Mass The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The reaction between hydrochloric acid and zinc. 2hcl + zn → zncl 2 + h 2. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Zn + hcl = zncl2 + h2 is. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED 52.3 g of Zinc metal is reacted with a solution containing 51.0 Zinc And Hydrochloric Acid Mass Hydrochloric acid + zinc → zinc chloride + hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction between zinc metal and the hydrochloric acid solution is an example. Zinc And Hydrochloric Acid Mass.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Mass Properties of zinc and specifics of its interactions with hcl. 2hcl + zn → zncl 2 + h 2. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Hydrochloric acid + zinc → zinc chloride + hydrogen. The reaction between hydrochloric acid and zinc. Oxidation/reduction, gas forming reaction, acid properties, net. Zinc And Hydrochloric Acid Mass.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid Zinc And Hydrochloric Acid Mass The reaction between hydrochloric acid and zinc. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. The hydrogen causes bubbling during the. The reaction between zinc metal and the hydrochloric acid solution is an. Zinc And Hydrochloric Acid Mass.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq Zinc And Hydrochloric Acid Mass Properties of zinc and specifics of its interactions with hcl. Hydrochloric acid + zinc → zinc chloride + hydrogen. The hydrogen causes bubbling during the. The reaction between hydrochloric acid and zinc. 2hcl + zn → zncl 2 + h 2. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: Zn + hcl = zncl2 + h2. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED Consider the reaction between zinc metal and hydrochloric acid Zinc And Hydrochloric Acid Mass The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Properties of zinc and specifics of its interactions with hcl. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. The reaction between hydrochloric acid and zinc. Oxidation/reduction, gas forming reaction, acid properties, net. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED Calculate the mass of hydrochloric acid (HCl) needed to react Zinc And Hydrochloric Acid Mass The reaction between hydrochloric acid and zinc. Properties of zinc and specifics of its interactions with hcl. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen causes bubbling during the. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: Hydrochloric acid + zinc → zinc chloride. Zinc And Hydrochloric Acid Mass.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc And Hydrochloric Acid Mass The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen causes bubbling during the. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zn + hcl = zncl2 + h2 is a. Zinc And Hydrochloric Acid Mass.
From melscience.com
The reaction between hydrochloric acid and zinc MEL Chemistry Zinc And Hydrochloric Acid Mass Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: Properties of zinc and specifics of its interactions with hcl. Hydrochloric acid + zinc → zinc chloride + hydrogen. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. 2hcl + zn → zncl 2 + h 2. The reaction. Zinc And Hydrochloric Acid Mass.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Zinc And Hydrochloric Acid Mass The hydrogen causes bubbling during the. Properties of zinc and specifics of its interactions with hcl. 2hcl + zn → zncl 2 + h 2. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. The reaction between hydrochloric acid and zinc. Hydrochloric acid + zinc → zinc chloride + hydrogen. The. Zinc And Hydrochloric Acid Mass.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc And Hydrochloric Acid Mass The reaction between hydrochloric acid and zinc. Properties of zinc and specifics of its interactions with hcl. 2hcl + zn → zncl 2 + h 2. The hydrogen causes bubbling during the. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Hydrochloric acid + zinc → zinc chloride + hydrogen. Oxidation/reduction,. Zinc And Hydrochloric Acid Mass.
From cameramath.com
[Solved] Assume 95.4 grams of zinc, Zn , reacts with hydrochloric acid Zinc And Hydrochloric Acid Mass The hydrogen causes bubbling during the. Hydrochloric acid + zinc → zinc chloride + hydrogen. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. The reaction between hydrochloric acid and zinc. Zn +. Zinc And Hydrochloric Acid Mass.
From www.nagwa.com
Question Video Understanding Oxidation and Reduction in the Reaction Zinc And Hydrochloric Acid Mass Hydrochloric acid + zinc → zinc chloride + hydrogen. Properties of zinc and specifics of its interactions with hcl. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction between hydrochloric acid and zinc. The hydrogen causes bubbling during the. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Mass Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. 2hcl + zn → zncl 2 + h 2. Properties of zinc and specifics of its interactions with hcl. The reaction between zinc metal and the hydrochloric acid solution is an example of a. Zinc And Hydrochloric Acid Mass.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc And Hydrochloric Acid Mass The hydrogen causes bubbling during the. Properties of zinc and specifics of its interactions with hcl. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. 2hcl + zn → zncl 2 + h. Zinc And Hydrochloric Acid Mass.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Zinc And Hydrochloric Acid Mass Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction between hydrochloric acid and zinc. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. The reaction between zinc metal and the hydrochloric acid solution is an example of a. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVEDZinc and magnesium metal each react with hydrochloric acid to Zinc And Hydrochloric Acid Mass The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. 2hcl + zn → zncl 2 + h 2. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction between hydrochloric acid and zinc. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED A 5 gram sample of zinc is added to hydrochloric acid. The Zinc And Hydrochloric Acid Mass Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. The hydrogen causes bubbling during the. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVEDZinc and magnesium metal each reacts with hydrochloric acid to Zinc And Hydrochloric Acid Mass Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The reaction between hydrochloric acid and zinc. Hydrochloric acid + zinc → zinc chloride + hydrogen. Properties of zinc and specifics of its interactions with hcl. The hydrogen causes bubbling. Zinc And Hydrochloric Acid Mass.
From quizlet.com
investigate reactions between dilute hydrochloric and sulfuric acids Zinc And Hydrochloric Acid Mass The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Hydrochloric acid + zinc → zinc chloride + hydrogen. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. The hydrogen causes bubbling during the. The reaction between hydrochloric acid and zinc. Properties of. Zinc And Hydrochloric Acid Mass.
From www.pdffiller.com
The reaction between hydrochloric acid and zinc MEL Chemistry Doc Zinc And Hydrochloric Acid Mass The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The reaction between hydrochloric acid and zinc. The hydrogen causes bubbling during the. 2hcl + zn → zncl 2 + h 2. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Properties of. Zinc And Hydrochloric Acid Mass.
From www.researchgate.net
The corrosion parameters of zinc in 1.0 N Hydrochloric acid containing Zinc And Hydrochloric Acid Mass The reaction between hydrochloric acid and zinc. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Hydrochloric acid + zinc → zinc chloride + hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen.. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED A 20.00 g mixture of magnesium and zinc metal reacting with Zinc And Hydrochloric Acid Mass Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The hydrogen causes bubbling during the. The reaction between hydrochloric acid and zinc. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Properties of zinc and specifics of its interactions with hcl. 2hcl + zn → zncl 2 +. Zinc And Hydrochloric Acid Mass.
From www.toppr.com
Zinc and hydrochloric acid react according to equation Zn(s) + 2HCl(aq Zinc And Hydrochloric Acid Mass Hydrochloric acid + zinc → zinc chloride + hydrogen. Properties of zinc and specifics of its interactions with hcl. 2hcl + zn → zncl 2 + h 2. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED Similar to magnesium, zinc also reacts with hydrochloric acid Zinc And Hydrochloric Acid Mass Hydrochloric acid + zinc → zinc chloride + hydrogen. The hydrogen causes bubbling during the. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction between hydrochloric acid and zinc.. Zinc And Hydrochloric Acid Mass.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Mass The hydrogen causes bubbling during the. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Properties of zinc and specifics of. Zinc And Hydrochloric Acid Mass.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc And Hydrochloric Acid Mass The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The hydrogen causes bubbling during the. 2hcl + zn → zncl 2 + h 2. The reaction between zinc metal and the hydrochloric acid solution is an example of a. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED Question 2 A student carried out some experiments between zinc Zinc And Hydrochloric Acid Mass Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. 2hcl + zn → zncl 2 + h 2. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Oxidation/reduction, gas forming reaction, acid properties, net. Zinc And Hydrochloric Acid Mass.
From www.youtube.com
Zinc and Hydrochloric Acid Mass Test YouTube Zinc And Hydrochloric Acid Mass Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Hydrochloric acid + zinc → zinc chloride + hydrogen. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen causes. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
SOLVED since 1982, pennies changed from being pure copper to zinc with Zinc And Hydrochloric Acid Mass The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Hydrochloric acid + zinc → zinc chloride + hydrogen. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one. Zinc And Hydrochloric Acid Mass.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc And Hydrochloric Acid Mass Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: Hydrochloric acid + zinc → zinc chloride + hydrogen. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and. Zinc And Hydrochloric Acid Mass.
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Zinc And Hydrochloric Acid Mass Hydrochloric acid + zinc → zinc chloride + hydrogen. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. The hydrogen causes bubbling during the. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic reactions description: The solid zinc lost mass because some. Zinc And Hydrochloric Acid Mass.
From askfilo.com
Zinc metal reacts with hydrochloric acid by the following reaction Zn(s).. Zinc And Hydrochloric Acid Mass Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride. 2hcl + zn → zncl 2 + h 2. The reaction between zinc metal and the. Zinc And Hydrochloric Acid Mass.
From slidesharetrick.blogspot.com
Zinc And Hydrochloric Acid slidesharetrick Zinc And Hydrochloric Acid Mass The reaction between hydrochloric acid and zinc. Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Hydrochloric acid + zinc → zinc chloride + hydrogen. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride.. Zinc And Hydrochloric Acid Mass.
From www.numerade.com
Zinc and magnesium metal each reacts with hydrochloric acid to make Zinc And Hydrochloric Acid Mass Zn + hcl = zncl2 + h2 is a single displacement (substitution) reaction where one mole of solid zinc [zn] and two moles of aqueous hydrogen. Hydrochloric acid + zinc → zinc chloride + hydrogen. The reaction between hydrochloric acid and zinc. The solid zinc lost mass because some of it reacted with the hydrochloric acid to form zinc chloride.. Zinc And Hydrochloric Acid Mass.