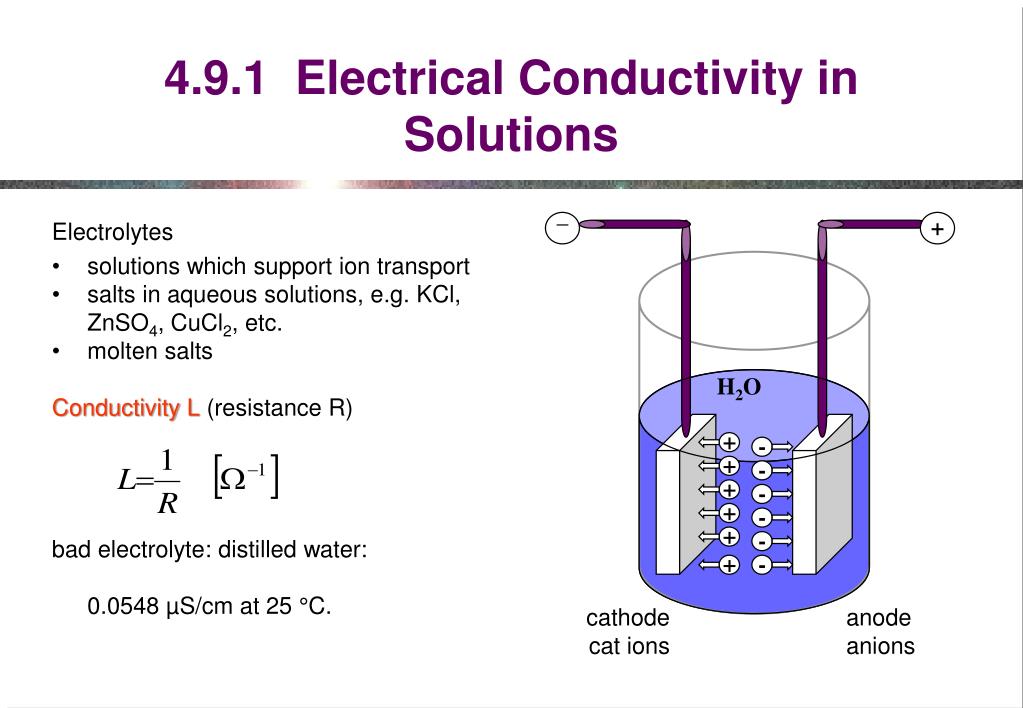
How Can Electrolyte Solutions Conduct Electricity . In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. The larger the concentration of ions, the better the solutions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. Ionic compounds conduct electricity when molten or. Reactive metals are extracted from their ores using electrolysis. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to.
from www.slideserve.com
The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Reactive metals are extracted from their ores using electrolysis. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. Ionic compounds conduct electricity when molten or. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. The larger the concentration of ions, the better the solutions.
PPT Electrochemistry PowerPoint Presentation, free download ID2012118
How Can Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. Reactive metals are extracted from their ores using electrolysis. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Ionic compounds conduct electricity when molten or. The larger the concentration of ions, the better the solutions. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a.
From www.britannica.com
Electric current in a solution of electrolytes studied Britannica How Can Electrolyte Solutions Conduct Electricity Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Ionic compounds conduct electricity. How Can Electrolyte Solutions Conduct Electricity.
From slideplayer.com
Electrolytes and pH. Electrolyte a substance that when dissolved in How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Reactive metals are extracted. How Can Electrolyte Solutions Conduct Electricity.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL How Can Electrolyte Solutions Conduct Electricity The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. In some cases, solutions prepared from covalent compounds conduct electricity because. How Can Electrolyte Solutions Conduct Electricity.
From www.slidegeeks.com
Electrolyte Solutions Conduct Electricity In Powerpoint And Google How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. The larger the concentration of ions, the better the solutions. Ionic compounds conduct electricity when molten or. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. An electrolyte solution. How Can Electrolyte Solutions Conduct Electricity.
From shaunmwilliams.com
Chapter 3 Presentation How Can Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Reactive metals are extracted from their ores using electrolysis. An electrolyte solution conducts electricity because of the movement of. How Can Electrolyte Solutions Conduct Electricity.
From slideplayer.com
WATER And Solution Formation ppt download How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Reactive metals are extracted from their ores using electrolysis. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. An electrolyte solution conducts electricity because of the movement of ions. How Can Electrolyte Solutions Conduct Electricity.
From www.pathwaystochemistry.com
Electrolytes and Nonelectrolytes Pathways to Chemistry How Can Electrolyte Solutions Conduct Electricity Reactive metals are extracted from their ores using electrolysis. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. An electrolyte solution conducts electricity because of the movement of. How Can Electrolyte Solutions Conduct Electricity.
From byjus.com
In an attempt to demonstrate electrical conductivity through an How Can Electrolyte Solutions Conduct Electricity Ionic compounds conduct electricity when molten or. The larger the concentration of ions, the better the solutions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In some cases, solutions prepared from covalent compounds conduct electricity because. How Can Electrolyte Solutions Conduct Electricity.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at How Can Electrolyte Solutions Conduct Electricity The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. Ionic compounds conduct electricity when molten or. In some cases, we find that solutions prepared from covalent. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Ionic compounds conduct electricity when molten or. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. In. How Can Electrolyte Solutions Conduct Electricity.
From studylib.net
Conductivity of Electrolytes in Solution How Can Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Reactive metals are extracted from their ores using electrolysis. Measure the conductivity of the following solutions, and classify each. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Chemistry 101 Chap. 4 PowerPoint Presentation, free download How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Ionic compounds conduct electricity when molten or. The larger the concentration of ions, the better the solutions. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. How Can Electrolyte Solutions Conduct Electricity.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps How Can Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. Reactive metals are extracted from their ores using electrolysis. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The word electrolyte was created by faraday in his famous. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Electrical Conductivity PowerPoint Presentation, free download How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. Measure the conductivity of the following solutions, and classify each solute as a strong. How Can Electrolyte Solutions Conduct Electricity.
From slideplayer.com
Chapter 15 = Solutions Solutions = homogeneous mixtures containing two How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to. How Can Electrolyte Solutions Conduct Electricity.
From www.electricity-magnetism.org
How do electrolytes conduct electricity? How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. Reactive metals are extracted from their ores using electrolysis. An electrolyte solution conducts electricity. How Can Electrolyte Solutions Conduct Electricity.
From chemistryfromscratch.org
V2.8 How Can Electrolyte Solutions Conduct Electricity The larger the concentration of ions, the better the solutions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. In some cases, solutions prepared from covalent compounds conduct electricity because. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID How Can Electrolyte Solutions Conduct Electricity The larger the concentration of ions, the better the solutions. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. An electrolyte. How Can Electrolyte Solutions Conduct Electricity.
From energyeducation.ca
Electrolyte Energy Education How Can Electrolyte Solutions Conduct Electricity The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with. How Can Electrolyte Solutions Conduct Electricity.
From slidetodoc.com
Lecture 9 Electrical conductivity of electrolytes solutions Prepared How Can Electrolyte Solutions Conduct Electricity The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. The larger the concentration of ions, the better the solutions. Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or. Measure the conductivity of the following solutions, and classify each solute. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT AP Chem Chapter 4 PowerPoint Presentation, free download ID2144903 How Can Electrolyte Solutions Conduct Electricity Reactive metals are extracted from their ores using electrolysis. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). In some cases, solutions prepared from covalent compounds conduct electricity. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Types of Solutes PowerPoint Presentation, free download ID9371021 How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. Reactive metals are extracted from their ores using electrolysis. Measure the. How Can Electrolyte Solutions Conduct Electricity.
From byjus.com
Conduction of Electricity in Liquids Electrolysis, Reduction at How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. The word electrolyte was created by faraday in his. How Can Electrolyte Solutions Conduct Electricity.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Reactive metals are extracted from their ores using electrolysis. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically. How Can Electrolyte Solutions Conduct Electricity.
From slideplayer.com
Day 71 Notes (Ch. 17 & 20) Electrolytes, Acids and Bases. ppt download How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react. How Can Electrolyte Solutions Conduct Electricity.
From studiousguy.com
9 Electrolyte Examples in Daily Life StudiousGuy How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Reactive metals are extracted from their ores using electrolysis. The larger the concentration of ions, the better the solutions. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. The. How Can Electrolyte Solutions Conduct Electricity.
From www.chemicals.co.uk
Why Does Potassium Iodide Solution Conduct Electricity? How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. Ionic compounds conduct electricity when molten or. The larger the concentration of ions, the better the solutions. The word electrolyte was created by faraday. How Can Electrolyte Solutions Conduct Electricity.
From exytxvzzy.blob.core.windows.net
How Electrolyte Solutions Conduct Electricity at Dewey Goodson blog How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. Measure. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Electrical conductivity of electrolyte’s solutions PowerPoint How Can Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Ionic compounds conduct electricity when molten or. The word electrolyte was created by faraday in his famous treatise of. How Can Electrolyte Solutions Conduct Electricity.
From slideplayer.com
Solutions. ppt download How Can Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to. How Can Electrolyte Solutions Conduct Electricity.
From slideplayer.com
ELECTROLYTES Substances that when dissolved in water will conduct How Can Electrolyte Solutions Conduct Electricity In some cases, solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to. Measure the conductivity of the following solutions, and classify each solute as a strong electrolyte, a weak electrolyte, or a. The larger the concentration of ions, the better the solutions. An electrolyte solution conducts electricity because of the movement of. How Can Electrolyte Solutions Conduct Electricity.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2012118 How Can Electrolyte Solutions Conduct Electricity In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. The larger the concentration of ions, the better the solutions. An electrolyte. How Can Electrolyte Solutions Conduct Electricity.
From www.teachoo.com
Do liquids conduct electricity? Class 8 Science Notes Teachoo How Can Electrolyte Solutions Conduct Electricity An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or. Measure the conductivity of the following solutions, and classify each solute as. How Can Electrolyte Solutions Conduct Electricity.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors How Can Electrolyte Solutions Conduct Electricity Reactive metals are extracted from their ores using electrolysis. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Measure the conductivity of the following solutions, and classify each solute as. How Can Electrolyte Solutions Conduct Electricity.
From www.scribd.com
CHM 130LL Electrolytes Lab Conduct Electricity. Ions Can Carry How Can Electrolyte Solutions Conduct Electricity Ionic compounds conduct electricity when molten or. The word electrolyte was created by faraday in his famous treatise of 1834, 1 when he was trying to explain why certain aqueous. An electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the better the solutions. Measure the conductivity of. How Can Electrolyte Solutions Conduct Electricity.