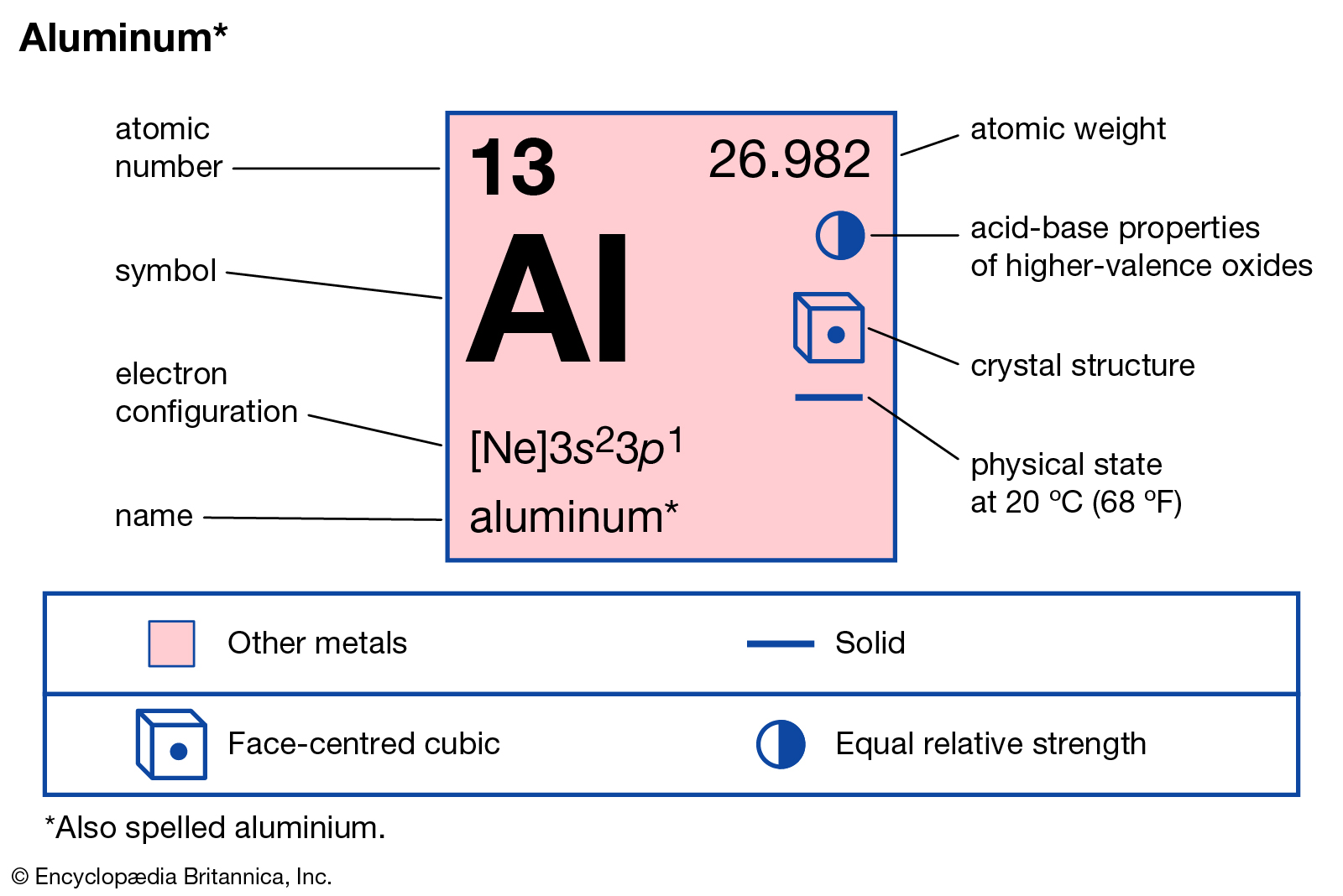
Aluminium Atomic Number And Electronic Configuration . Atomic weight of aluminium is 26.98 amu. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. Aluminium, a lightweight metal, holds a significant place in various industries. The ground state electronic configuration of neutral aluminium is [ne]. Its atomic number is 13,. Aluminium has 13 electrons in total. Aluminium has 13 electrons and 14 neutrons. To find the electron configuration of an atom, you first need to know the number of electrons that. The electron configuration for aluminum is. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminum is one of the elements that make up the periodic table, which is distinguished by. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The proton number is also 13.
from www.enfo.hu
Aluminium has 13 electrons and 14 neutrons. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. Aluminium, a lightweight metal, holds a significant place in various industries. The proton number is also 13. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Atomic weight of aluminium is 26.98 amu. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. The ground state electronic configuration of neutral aluminium is [ne]. Its atomic number is 13,.
Aluminium ENvironmental inFOrmation
Aluminium Atomic Number And Electronic Configuration The proton number is also 13. Aluminium, a lightweight metal, holds a significant place in various industries. The electron configuration for aluminum is. The ground state electronic configuration of neutral aluminium is [ne]. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The proton number is also 13. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Aluminium has 13 electrons in total. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. Its atomic number is 13,. Aluminum is one of the elements that make up the periodic table, which is distinguished by. Atomic weight of aluminium is 26.98 amu. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Aluminium has 13 electrons and 14 neutrons. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1.
From ar.inspiredpencil.com
Aluminium Atomic Structure Aluminium Atomic Number And Electronic Configuration Aluminium, a lightweight metal, holds a significant place in various industries. Atomic weight of aluminium is 26.98 amu. The proton number is also 13. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. In order to write the aluminium electron configuration we first need to know. Aluminium Atomic Number And Electronic Configuration.
From sciencenotes.org
Aluminum Atom Science Notes and Projects Aluminium Atomic Number And Electronic Configuration Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). The electron configuration for aluminum is. Atomic weight of aluminium is 26.98 amu. Its atomic number is 13,. The proton number is also 13. Aluminium has 13 electrons and 14 neutrons. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Sources, facts, uses, scarcity (sri), podcasts,. Aluminium Atomic Number And Electronic Configuration.
From www.youtube.com
Al 3+ Electron Configuration (Aluminum Ion) YouTube Aluminium Atomic Number And Electronic Configuration The ground state electronic configuration of neutral aluminium is [ne]. The electron configuration for aluminum is. Atomic weight of aluminium is 26.98 amu. The proton number is also 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. To find the electron configuration of an atom, you first need to know the number of electrons that. Aluminium has 13 electrons and. Aluminium Atomic Number And Electronic Configuration.
From www.alamy.com
Aluminium (Al). Diagram of the nuclear composition and electron Aluminium Atomic Number And Electronic Configuration In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The proton number is also 13. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. The electron configuration for aluminum is. Aluminum is one of the elements that make up the periodic table, which. Aluminium Atomic Number And Electronic Configuration.
From periodictable.me
How Can We Find Electron Configuration For Aluminium (Al) Aluminium Atomic Number And Electronic Configuration In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Its atomic number is 13,. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Aluminium atoms have 13 electrons and the shell structure is 2.8.3. To find the electron configuration. Aluminium Atomic Number And Electronic Configuration.
From valenceelectrons.com
Electron Configuration for Aluminum (Al, Al3+ ion) Aluminium Atomic Number And Electronic Configuration The electron configuration for aluminum is. Aluminium, a lightweight metal, holds a significant place in various industries. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. In order to write the aluminium electron configuration we first need. Aluminium Atomic Number And Electronic Configuration.
From www.numerade.com
table 22 expected electron configurations for some common elementsa Aluminium Atomic Number And Electronic Configuration In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The ground state electronic configuration of neutral aluminium is [ne]. Aluminium, a lightweight metal, holds a significant place in various industries. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. Aluminium has 13 electrons. Aluminium Atomic Number And Electronic Configuration.
From www.alamy.com
Aluminium (Al). Diagram of the nuclear composition, electron Aluminium Atomic Number And Electronic Configuration Aluminum is one of the elements that make up the periodic table, which is distinguished by. Atomic weight of aluminium is 26.98 amu. The proton number is also 13. The electron configuration for aluminum is. The ground state electronic configuration of neutral aluminium is [ne]. Aluminium has 13 electrons in total. To find the electron configuration of an atom, you. Aluminium Atomic Number And Electronic Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Aluminum Aluminium Atomic Number And Electronic Configuration The proton number is also 13. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. Aluminium, a lightweight metal, holds a significant place in various industries. Its atomic number is 13,. Atomic weight of aluminium is 26.98 amu. The electron configuration for aluminum is. Aluminium. Aluminium Atomic Number And Electronic Configuration.
From periodictable.me
How Can We Find Electron Configuration For Aluminium (Al) Aluminium Atomic Number And Electronic Configuration Aluminium, a lightweight metal, holds a significant place in various industries. The proton number is also 13. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The ground state electronic configuration of neutral aluminium is [ne]. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Its atomic number is 13,. In order to write the aluminium electron configuration we first need to. Aluminium Atomic Number And Electronic Configuration.
From imgbin.com
Aluminium Electron Shell Electron Configuration Chemical Element Atom Aluminium Atomic Number And Electronic Configuration The ground state electronic configuration of neutral aluminium is [ne]. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Atomic weight of aluminium is 26.98 amu. Aluminium, a lightweight metal,. Aluminium Atomic Number And Electronic Configuration.
From valenceelectrons.com
Protons, Neutrons, Electrons for Aluminum (Al, Al3+) Aluminium Atomic Number And Electronic Configuration To find the electron configuration of an atom, you first need to know the number of electrons that. The ground state electronic configuration of neutral aluminium is [ne]. Aluminium has 13 electrons and 14 neutrons. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The electron configuration for aluminum is. Aluminum is one. Aluminium Atomic Number And Electronic Configuration.
From valenceelectrons.com
How to Write the Electron Configuration for Aluminium (Al)? Aluminium Atomic Number And Electronic Configuration To find the electron configuration of an atom, you first need to know the number of electrons that. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. Aluminium has 13 electrons in total. Aluminium. Aluminium Atomic Number And Electronic Configuration.
From brainly.in
Atomic structure of aluminum Brainly.in Aluminium Atomic Number And Electronic Configuration The electron configuration for aluminum is. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminium has 13 electrons and 14 neutrons. Atomic weight of aluminium is 26.98 amu. To find the electron configuration of an atom, you first need to know the number of electrons. Aluminium Atomic Number And Electronic Configuration.
From smk-tpz-web-api-1325663342.ap-south-1.elb.amazonaws.com
Al Aluminium Element Information Facts, Properties, Trends, Uses and Aluminium Atomic Number And Electronic Configuration The proton number is also 13. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Aluminum is one of the elements that make up the periodic table, which is. Aluminium Atomic Number And Electronic Configuration.
From mavink.com
Aluminum Shell Diagram Aluminium Atomic Number And Electronic Configuration ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. The ground state electronic configuration of neutral aluminium is [ne]. Aluminum is one of the elements that make up the periodic table, which is distinguished by. Aluminium has 13 electrons and 14 neutrons. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Atomic weight of aluminium. Aluminium Atomic Number And Electronic Configuration.
From newtondesk.com
Aluminium Al (Element 13) of Periodic Table Elements FlashCards Aluminium Atomic Number And Electronic Configuration The electron configuration for aluminum is. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Its atomic number is 13,. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. Aluminium. Aluminium Atomic Number And Electronic Configuration.
From www.sciencephoto.com
Aluminium, atomic structure Stock Image C013/1526 Science Photo Aluminium Atomic Number And Electronic Configuration Aluminium atoms have 13 electrons and the shell structure is 2.8.3. The ground state electronic configuration of neutral aluminium is [ne]. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminium has 13 electrons in total. Its atomic number is 13,. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. Aluminium Atomic Number And Electronic Configuration.
From www.vectorstock.com
Diagram representation of the element aluminium Vector Image Aluminium Atomic Number And Electronic Configuration Its atomic number is 13,. The electron configuration for aluminum is. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). The ground state electronic configuration of neutral aluminium is [ne]. Aluminium has 13 electrons and 14 neutrons. Aluminium has 13 electrons in total. In order to write the aluminium electron configuration we first need. Aluminium Atomic Number And Electronic Configuration.
From material-properties.org
Aluminium Protons Neutrons Electrons Electron Configuration Aluminium Atomic Number And Electronic Configuration In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The ground state electronic configuration of neutral aluminium is [ne]. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). The electron configuration for aluminum is. Aluminium has 13 electrons and. Aluminium Atomic Number And Electronic Configuration.
From www.youtube.com
Atomic Structure (Bohr Model) for Aluminum (Al) YouTube Aluminium Atomic Number And Electronic Configuration The proton number is also 13. To find the electron configuration of an atom, you first need to know the number of electrons that. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminium, a lightweight metal, holds a significant place in various industries. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. The electron configuration for aluminum is.. Aluminium Atomic Number And Electronic Configuration.
From www.pinterest.com
FileElectron shell 013 Aluminum.svg Wikimedia Commons Atom diagram Aluminium Atomic Number And Electronic Configuration An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Aluminium has 13 electrons and 14 neutrons. Aluminium can relatively easily surrender. Aluminium Atomic Number And Electronic Configuration.
From valenceelectrons.com
How to Write the Electron Configuration for Aluminium (Al)? Aluminium Atomic Number And Electronic Configuration Aluminium atoms have 13 electrons and the shell structure is 2.8.3. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble gas configuration. The electron configuration for aluminum is. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Aluminium has 13 electrons. Aluminium Atomic Number And Electronic Configuration.
From www.alamy.com
A Aluminium atom diagram Stock Vector Image & Art Alamy Aluminium Atomic Number And Electronic Configuration Its atomic number is 13,. Aluminum is one of the elements that make up the periodic table, which is distinguished by. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). The electron configuration for aluminum is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminium, a lightweight metal, holds a significant place in various. Aluminium Atomic Number And Electronic Configuration.
From www.dreamstime.com
Model of aluminium atom stock vector. Illustration of science 164474877 Aluminium Atomic Number And Electronic Configuration To find the electron configuration of an atom, you first need to know the number of electrons that. Atomic weight of aluminium is 26.98 amu. Aluminium, a lightweight metal, holds a significant place in various industries. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Aluminium has 13 electrons in total. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminium has. Aluminium Atomic Number And Electronic Configuration.
From mavink.com
Aluminum Shell Diagram Aluminium Atomic Number And Electronic Configuration The electron configuration for aluminum is. Aluminium has 13 electrons in total. Aluminium has 13 electrons and 14 neutrons. Aluminium, a lightweight metal, holds a significant place in various industries. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a stable noble. Aluminium Atomic Number And Electronic Configuration.
From www.alamy.com
Diagram of the nuclear composition, electron configuration, and valence Aluminium Atomic Number And Electronic Configuration Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. To find the electron configuration of an atom, you first need to know the number of electrons that. The ground state electronic configuration of neutral aluminium is [ne]. The electron configuration for aluminum is. Aluminium has 13 electrons in total. Aluminium has 13 electrons and 14 neutrons. The proton number is also. Aluminium Atomic Number And Electronic Configuration.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Aluminium Atomic Number And Electronic Configuration ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. The proton number is also 13. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Atomic weight of aluminium is 26.98 amu. Aluminum is one of the elements that make up the periodic table, which. Aluminium Atomic Number And Electronic Configuration.
From ar.inspiredpencil.com
Aluminium Electron Configuration Aluminium Atomic Number And Electronic Configuration Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). Atomic weight of aluminium is 26.98 amu. The ground state electronic configuration of neutral aluminium is [ne]. The electron configuration for aluminum is. Aluminium, a lightweight metal, holds a significant place in various industries. The proton number. Aluminium Atomic Number And Electronic Configuration.
From topblogtenz.com
Aluminum Orbital diagram, Electron configuration, and Valence electrons Aluminium Atomic Number And Electronic Configuration The electron configuration for aluminum is. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The ground state electronic configuration of neutral aluminium is [ne]. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Atomic weight of aluminium. Aluminium Atomic Number And Electronic Configuration.
From www.alamy.com
3d render of atom structure of aluminum isolated over white background Aluminium Atomic Number And Electronic Configuration Aluminium can relatively easily surrender its three outermost electrons in many chemical reactions (see below). ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. To find the electron configuration of an atom, you first need to know the number of electrons that. Its atomic number is 13,. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The ground state electronic configuration of. Aluminium Atomic Number And Electronic Configuration.
From www.alamy.com
Al Aluminium Chemical Element Periodic Table. Single vector Aluminium Atomic Number And Electronic Configuration Aluminum is one of the elements that make up the periodic table, which is distinguished by. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. Its atomic number is 13,. The ground state electronic configuration of neutral aluminium is [ne]. In order to write the aluminium electron configuration we first need to know the number of electrons for. Aluminium Atomic Number And Electronic Configuration.
From aluminumgenjin.blogspot.com
Aluminum Electron Configuration For Aluminum Aluminium Atomic Number And Electronic Configuration In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. The ground state electronic configuration of neutral aluminium is [ne]. Aluminium atoms have 13 electrons and the shell structure is 2.8.3. Its atomic number is 13,. Aluminium has 13 electrons in total. The electron configuration for. Aluminium Atomic Number And Electronic Configuration.
From www.enfo.hu
Aluminium ENvironmental inFOrmation Aluminium Atomic Number And Electronic Configuration To find the electron configuration of an atom, you first need to know the number of electrons that. Aluminium has 13 electrons and 14 neutrons. The electron configuration for aluminum is. ⬆️⬆️⬆️ the electron configuration of aluminum is 1s22s22p63s23p1. Atomic weight of aluminium is 26.98 amu. Aluminium, a lightweight metal, holds a significant place in various industries. Sources, facts, uses,. Aluminium Atomic Number And Electronic Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Aluminum Aluminium Atomic Number And Electronic Configuration Aluminium, a lightweight metal, holds a significant place in various industries. In order to write the aluminium electron configuration we first need to know the number of electrons for the al atom (there are 13. Its atomic number is 13,. An aluminium atom has 13 electrons, arranged in an electron configuration of [ne] 3s2 3p1, with three electrons beyond a. Aluminium Atomic Number And Electronic Configuration.