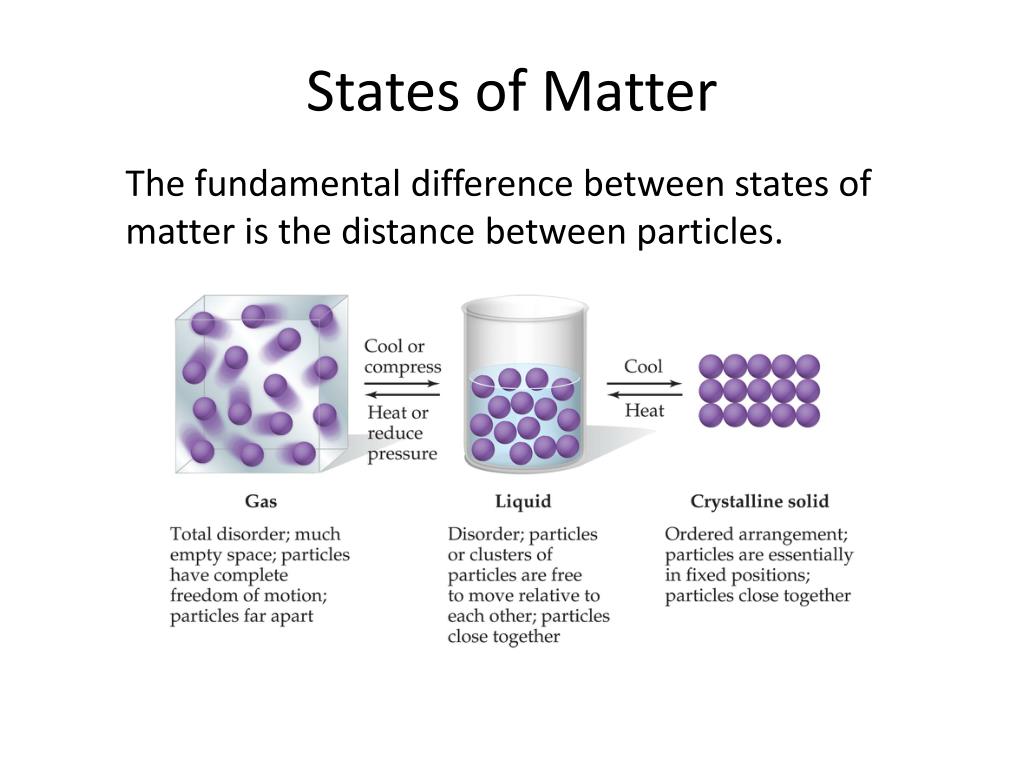
Solid Liquid Gas Intermolecular Forces . Attractive forces between molecules intramolecular forces: Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Gases or liquids at room temperature from. These are forces between molecules. In general covalent bonds determine: In contrast to intramolecular forces, such. Physical properties of substances are. Physical properties of liquids and solids are due to intermolecular forces. In a gas, they move. Hold atoms together in a molecule. In a liquid, they move past each other but remain in essentially constant contact;
from www.slideserve.com
In general covalent bonds determine: Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; Gases or liquids at room temperature from. In a gas, they move. Physical properties of substances are. Physical properties of liquids and solids are due to intermolecular forces. Hold atoms together in a molecule. Attractive forces between molecules intramolecular forces: The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In a liquid, they move past each other but remain in essentially constant contact;
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint
Solid Liquid Gas Intermolecular Forces The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In a gas, they move. Physical properties of liquids and solids are due to intermolecular forces. Attractive forces between molecules intramolecular forces: In contrast to intramolecular forces, such. In a liquid, they move past each other but remain in essentially constant contact; Hold atoms together in a molecule. Gases or liquids at room temperature from. In general covalent bonds determine: Physical properties of substances are. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. These are forces between molecules.
From www.youtube.com
Intermolecular forces Solid liquid gas matter in our surroundings Solid Liquid Gas Intermolecular Forces The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Physical properties of liquids and solids are due to intermolecular forces. Physical properties of substances are. In contrast to intramolecular forces, such. Gases or liquids at room temperature from. In a gas, they move. Attractive forces between molecules intramolecular forces: In general. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Solid Liquid Gas Intermolecular Forces Gases or liquids at room temperature from. Physical properties of liquids and solids are due to intermolecular forces. Physical properties of substances are. Hold atoms together in a molecule. Attractive forces between molecules intramolecular forces: In a liquid, they move past each other but remain in essentially constant contact; In general covalent bonds determine: Particles in a solid vibrate about. Solid Liquid Gas Intermolecular Forces.
From opentextbc.ca
10.1 Intermolecular Forces Chemistry Solid Liquid Gas Intermolecular Forces In a liquid, they move past each other but remain in essentially constant contact; Attractive forces between molecules intramolecular forces: In contrast to intramolecular forces, such. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; Physical properties of substances are. Hold atoms together in a molecule. Physical properties of liquids and. Solid Liquid Gas Intermolecular Forces.
From schoolbag.info
For a given substance, do you expect the density of the substance in Solid Liquid Gas Intermolecular Forces Gases or liquids at room temperature from. Physical properties of liquids and solids are due to intermolecular forces. Hold atoms together in a molecule. In a gas, they move. Physical properties of substances are. Attractive forces between molecules intramolecular forces: In a liquid, they move past each other but remain in essentially constant contact; Particles in a solid vibrate about. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT SOLID LIQUID GAS PowerPoint Presentation, free download ID6902694 Solid Liquid Gas Intermolecular Forces Gases or liquids at room temperature from. Physical properties of liquids and solids are due to intermolecular forces. Attractive forces between molecules intramolecular forces: Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; In a liquid, they move past each other but remain in essentially constant contact; Hold atoms together in. Solid Liquid Gas Intermolecular Forces.
From slideplayer.com
Intermolecular Forces ppt download Solid Liquid Gas Intermolecular Forces In a liquid, they move past each other but remain in essentially constant contact; These are forces between molecules. In general covalent bonds determine: Physical properties of liquids and solids are due to intermolecular forces. Hold atoms together in a molecule. Physical properties of substances are. The properties of liquids are intermediate between those of gases and solids, but are. Solid Liquid Gas Intermolecular Forces.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid Liquid Gas Intermolecular Forces Physical properties of substances are. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. These are forces between molecules. Physical properties of liquids and solids are due to intermolecular forces. In a gas, they move. Attractive forces between molecules intramolecular forces: Particles in a solid vibrate about fixed positions and do. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Solid Liquid Gas Intermolecular Forces In general covalent bonds determine: Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; In a gas, they move. Gases or liquids at room temperature from. Physical properties of liquids and solids are due to intermolecular forces. The properties of liquids are intermediate between those of gases and solids, but are. Solid Liquid Gas Intermolecular Forces.
From www.studocu.com
Chapter 12 Liquids and Solids chapter 12 liquids and solids Solid Liquid Gas Intermolecular Forces In a liquid, they move past each other but remain in essentially constant contact; These are forces between molecules. Physical properties of liquids and solids are due to intermolecular forces. In general covalent bonds determine: In contrast to intramolecular forces, such. Hold atoms together in a molecule. Gases or liquids at room temperature from. Particles in a solid vibrate about. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Solid Liquid Gas Intermolecular Forces In a gas, they move. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In a liquid, they move past each other but remain in essentially constant contact; Gases or liquids at room. Solid Liquid Gas Intermolecular Forces.
From www.yumpu.com
Chapter 12 Liquids, Solids, and Intermolecular Forces Solid Liquid Gas Intermolecular Forces Hold atoms together in a molecule. These are forces between molecules. In contrast to intramolecular forces, such. Physical properties of substances are. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; Attractive forces between molecules intramolecular forces: In a gas, they move. Physical properties of liquids and solids are due to. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation, free download ID Solid Liquid Gas Intermolecular Forces Hold atoms together in a molecule. Attractive forces between molecules intramolecular forces: Gases or liquids at room temperature from. Physical properties of substances are. In a gas, they move. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In a liquid, they move past each other but remain in essentially constant. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces PowerPoint Solid Liquid Gas Intermolecular Forces In general covalent bonds determine: In contrast to intramolecular forces, such. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; Gases or liquids at room temperature from. In a liquid, they move past. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Chapter 12 Intermolecular Attractions and the Properties of Solid Liquid Gas Intermolecular Forces These are forces between molecules. In a liquid, they move past each other but remain in essentially constant contact; Physical properties of liquids and solids are due to intermolecular forces. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; In general covalent bonds determine: In contrast to intramolecular forces, such. Physical. Solid Liquid Gas Intermolecular Forces.
From www.slideshare.net
Intermolecular forces (liquids and solids) Solid Liquid Gas Intermolecular Forces Physical properties of substances are. Attractive forces between molecules intramolecular forces: In a liquid, they move past each other but remain in essentially constant contact; Hold atoms together in a molecule. In contrast to intramolecular forces, such. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In general covalent bonds determine:. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Chapter 9 Intermolecular Forces, Liquids, and Solids PowerPoint Solid Liquid Gas Intermolecular Forces Gases or liquids at room temperature from. In contrast to intramolecular forces, such. These are forces between molecules. Attractive forces between molecules intramolecular forces: In a gas, they move. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; In a liquid, they move past each other but remain in essentially constant. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces Solid Liquid Gas Intermolecular Forces Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; Gases or liquids at room temperature from. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Attractive forces between molecules intramolecular forces: In contrast to intramolecular forces, such. In a liquid, they move. Solid Liquid Gas Intermolecular Forces.
From www.youtube.com
Intermolecular Forces of Liquids and Solids YouTube Solid Liquid Gas Intermolecular Forces Gases or liquids at room temperature from. Hold atoms together in a molecule. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In a gas, they move. These are forces between molecules. Attractive forces between molecules intramolecular forces: Physical properties of substances are. In contrast to intramolecular forces, such. Particles in. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Solid Liquid Gas Intermolecular Forces In a gas, they move. Physical properties of liquids and solids are due to intermolecular forces. Physical properties of substances are. In general covalent bonds determine: The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Attractive forces between molecules intramolecular forces: Particles in a solid vibrate about fixed positions and do. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids and Solids PowerPoint Solid Liquid Gas Intermolecular Forces Physical properties of substances are. Attractive forces between molecules intramolecular forces: In contrast to intramolecular forces, such. These are forces between molecules. Physical properties of liquids and solids are due to intermolecular forces. In a liquid, they move past each other but remain in essentially constant contact; In general covalent bonds determine: In a gas, they move. Gases or liquids. Solid Liquid Gas Intermolecular Forces.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces Solid Liquid Gas Intermolecular Forces In a liquid, they move past each other but remain in essentially constant contact; Gases or liquids at room temperature from. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; Physical properties of substances are. Attractive forces between molecules intramolecular forces: In a gas, they move. These are forces between molecules.. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces, Liquids, and Solids PowerPoint Solid Liquid Gas Intermolecular Forces In general covalent bonds determine: Hold atoms together in a molecule. Gases or liquids at room temperature from. In contrast to intramolecular forces, such. In a gas, they move. Attractive forces between molecules intramolecular forces: In a liquid, they move past each other but remain in essentially constant contact; Physical properties of substances are. Particles in a solid vibrate about. Solid Liquid Gas Intermolecular Forces.
From dokumen.tips
(PPT) Intermolecular Forces Important differences between gases, solids Solid Liquid Gas Intermolecular Forces These are forces between molecules. Physical properties of substances are. In general covalent bonds determine: In contrast to intramolecular forces, such. In a liquid, they move past each other but remain in essentially constant contact; Gases or liquids at room temperature from. Hold atoms together in a molecule. The properties of liquids are intermediate between those of gases and solids,. Solid Liquid Gas Intermolecular Forces.
From stock.adobe.com
Three states of matter show intermolecular forces. Vector diagram with Solid Liquid Gas Intermolecular Forces In a liquid, they move past each other but remain in essentially constant contact; In general covalent bonds determine: The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Hold atoms together in a molecule. Particles in a solid vibrate about fixed positions and do not generally move in relation to one. Solid Liquid Gas Intermolecular Forces.
From www.slideshare.net
Chapter 11 Lecture Intermolecular Forces, Liquids, & Solids Solid Liquid Gas Intermolecular Forces Attractive forces between molecules intramolecular forces: These are forces between molecules. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; In a liquid, they move past each other but remain in essentially constant. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Chapter 11 Liquids, Solids, and Intermolecular Forces Solid Liquid Gas Intermolecular Forces The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In general covalent bonds determine: Attractive forces between molecules intramolecular forces: Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; In contrast to intramolecular forces, such. Gases or liquids at room temperature from.. Solid Liquid Gas Intermolecular Forces.
From slideplayer.com
Liquids, Solids, and Intermolecular Forces ppt download Solid Liquid Gas Intermolecular Forces These are forces between molecules. Hold atoms together in a molecule. Physical properties of substances are. In general covalent bonds determine: Gases or liquids at room temperature from. In a liquid, they move past each other but remain in essentially constant contact; In contrast to intramolecular forces, such. Attractive forces between molecules intramolecular forces: In a gas, they move. Solid Liquid Gas Intermolecular Forces.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning Solid Liquid Gas Intermolecular Forces In general covalent bonds determine: Physical properties of substances are. In a liquid, they move past each other but remain in essentially constant contact; Gases or liquids at room temperature from. Hold atoms together in a molecule. Physical properties of liquids and solids are due to intermolecular forces. Particles in a solid vibrate about fixed positions and do not generally. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT SOLID LIQUID GAS PowerPoint Presentation, free download ID6902694 Solid Liquid Gas Intermolecular Forces In a liquid, they move past each other but remain in essentially constant contact; Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. In contrast to intramolecular forces, such. Hold atoms together in. Solid Liquid Gas Intermolecular Forces.
From chem.libretexts.org
Intro to Phases and Intermolecular Forces Chemistry LibreTexts Solid Liquid Gas Intermolecular Forces In contrast to intramolecular forces, such. Attractive forces between molecules intramolecular forces: In a liquid, they move past each other but remain in essentially constant contact; Physical properties of substances are. These are forces between molecules. In a gas, they move. Hold atoms together in a molecule. Gases or liquids at room temperature from. Particles in a solid vibrate about. Solid Liquid Gas Intermolecular Forces.
From www.slideserve.com
PPT Intermolecular Forces Phases of Matter & Colligative Properties Solid Liquid Gas Intermolecular Forces Hold atoms together in a molecule. In a liquid, they move past each other but remain in essentially constant contact; Physical properties of substances are. In a gas, they move. Gases or liquids at room temperature from. In general covalent bonds determine: Attractive forces between molecules intramolecular forces: In contrast to intramolecular forces, such. The properties of liquids are intermediate. Solid Liquid Gas Intermolecular Forces.
From www.tutorix.com
Matter exists in three physical forms solid liquid Tutorix Solid Liquid Gas Intermolecular Forces In a liquid, they move past each other but remain in essentially constant contact; These are forces between molecules. In contrast to intramolecular forces, such. Physical properties of liquids and solids are due to intermolecular forces. Physical properties of substances are. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; Gases. Solid Liquid Gas Intermolecular Forces.
From www.jove.com
11340.jpg Solid Liquid Gas Intermolecular Forces Physical properties of substances are. These are forces between molecules. In general covalent bonds determine: Gases or liquids at room temperature from. Hold atoms together in a molecule. In contrast to intramolecular forces, such. Particles in a solid vibrate about fixed positions and do not generally move in relation to one another; In a liquid, they move past each other. Solid Liquid Gas Intermolecular Forces.
From slideplayer.com
Intermolecular forces ppt download Solid Liquid Gas Intermolecular Forces These are forces between molecules. Physical properties of liquids and solids are due to intermolecular forces. Hold atoms together in a molecule. In general covalent bonds determine: In a gas, they move. The properties of liquids are intermediate between those of gases and solids, but are more similar to solids. Physical properties of substances are. Particles in a solid vibrate. Solid Liquid Gas Intermolecular Forces.
From pdfslide.net
(PPTX) Solids, liquids and gases Solids, liquids, and gases are held Solid Liquid Gas Intermolecular Forces Physical properties of substances are. Hold atoms together in a molecule. In contrast to intramolecular forces, such. Physical properties of liquids and solids are due to intermolecular forces. In general covalent bonds determine: In a gas, they move. In a liquid, they move past each other but remain in essentially constant contact; Particles in a solid vibrate about fixed positions. Solid Liquid Gas Intermolecular Forces.