What Is Q Heat Transfer . Heat is transferred between two atoms or molecules in direct contact. Common units for heat ˙q transfer rate is btu/hr. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Sometimes it is important to. The symbol c stands for the specific heat (also called “. The quantitative relationship between heat transfer and temperature change contains all three factors: When we want to know the change in enthalpy. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The energy transfer is always from. \[q=m c \delta t, \nonumber \] where \(q\) is the. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The rate at which heat is transferred is represented by the symbol q.
from www.chegg.com
Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Heat is transferred between two atoms or molecules in direct contact. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The symbol c stands for the specific heat (also called “. \[q=m c \delta t, \nonumber \] where \(q\) is the. Common units for heat ˙q transfer rate is btu/hr. The rate at which heat is transferred is represented by the symbol q. Sometimes it is important to. The quantitative relationship between heat transfer and temperature change contains all three factors:
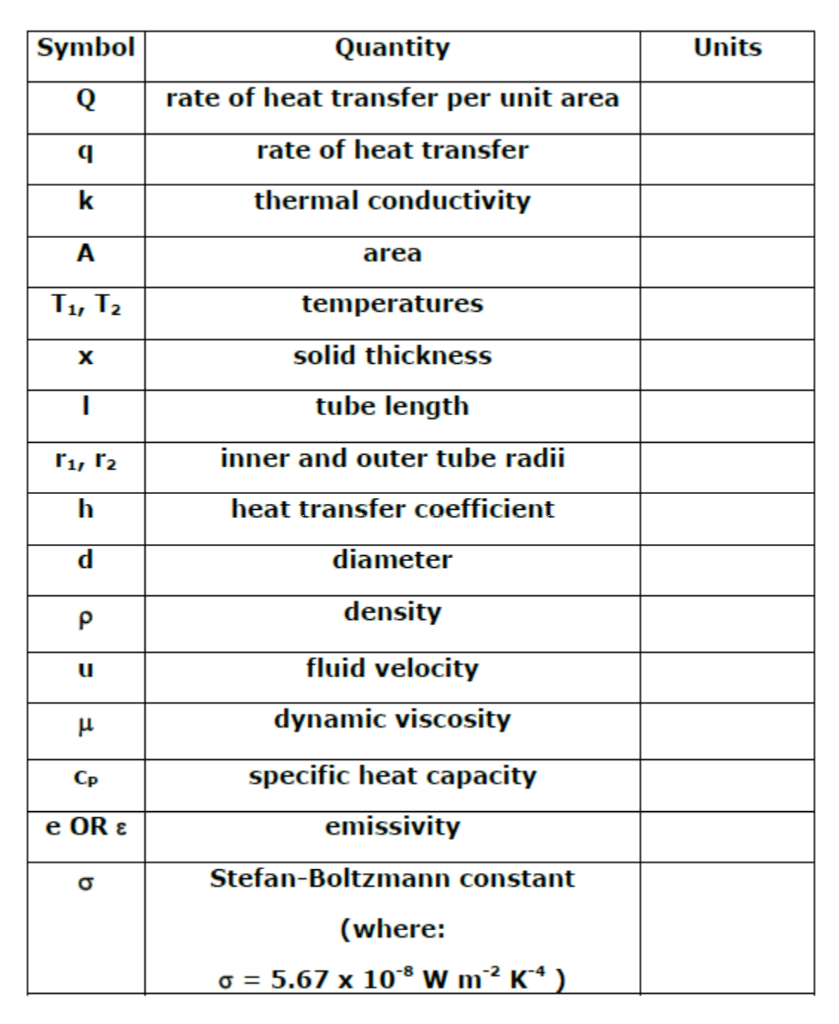
Solved Units Symbol Q Quantity rate of heat transfer per
What Is Q Heat Transfer The rate at which heat is transferred is represented by the symbol q. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The symbol c stands for the specific heat (also called “. When we want to know the change in enthalpy. The quantitative relationship between heat transfer and temperature change contains all three factors: Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Sometimes it is important to. The energy transfer is always from. Common units for heat ˙q transfer rate is btu/hr. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. The rate at which heat is transferred is represented by the symbol q. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Heat is transferred between two atoms or molecules in direct contact. \[q=m c \delta t, \nonumber \] where \(q\) is the.
From www.slideserve.com
PPT Heat Transfer PowerPoint Presentation, free download ID4604972 What Is Q Heat Transfer Sometimes it is important to. The quantitative relationship between heat transfer and temperature change contains all three factors: The energy transfer is always from. \[q=m c \delta t, \nonumber \] where \(q\) is the. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Heat is an energy transfer, whereas enthalpy is. What Is Q Heat Transfer.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free What Is Q Heat Transfer Sometimes it is important to. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The rate at which heat is transferred is represented by the symbol q. When we want to know the change in enthalpy. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,.. What Is Q Heat Transfer.
From www.youtube.com
Calculating thermal energy changes Q=mcdT YouTube What Is Q Heat Transfer \[q=m c \delta t, \nonumber \] where \(q\) is the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The energy transfer is always from. When we want to know the change in enthalpy. The rate at which heat is transferred. What Is Q Heat Transfer.
From www.nuclear-power.com
Heat Transfer Definition, Mechanisms & Application What Is Q Heat Transfer When we want to know the change in enthalpy. The quantitative relationship between heat transfer and temperature change contains all three factors: Common units for heat ˙q transfer rate is btu/hr. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Sometimes it is important to. Heat is transferred between two atoms or molecules. What Is Q Heat Transfer.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free What Is Q Heat Transfer Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The transfer occurs when agitated molecules at high temperatures strike slower molecules at. What Is Q Heat Transfer.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free What Is Q Heat Transfer When we want to know the change in enthalpy. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. The energy. What Is Q Heat Transfer.
From www.slideserve.com
PPT Heat (q) PowerPoint Presentation, free download ID1551407 What Is Q Heat Transfer Heat is transferred between two atoms or molecules in direct contact. The symbol c stands for the specific heat (also called “. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The rate at which heat is transferred is represented by the symbol q. The energy transfer is always from. Common units for. What Is Q Heat Transfer.
From studylib.net
What is Heat Transfer? What Is Q Heat Transfer Common units for heat ˙q transfer rate is btu/hr. Heat is transferred between two atoms or molecules in direct contact. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. Sometimes it is important to. When we want to know the change in enthalpy. Q = mcδt, where q is the symbol for heat transfer. What Is Q Heat Transfer.
From courses.lumenlearning.com
Temperature Change and Heat Capacity Physics What Is Q Heat Transfer \[q=m c \delta t, \nonumber \] where \(q\) is the. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. The quantitative relationship between heat transfer and temperature change contains all three factors: Common units for heat ˙q transfer rate is btu/hr. Heat transfer is the movement of heat due to a temperature difference between. What Is Q Heat Transfer.
From quizlet.com
Heat Transfer Diagram Quizlet What Is Q Heat Transfer Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The quantitative relationship between heat transfer and temperature change contains all three factors: Common units for heat ˙q transfer rate is btu/hr. When we want to know the change in enthalpy. Heat is transferred between two atoms or molecules in direct contact.. What Is Q Heat Transfer.
From mepacademy.com
How Heat Transfer Works MEP Academy What Is Q Heat Transfer Common units for heat ˙q transfer rate is btu/hr. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. The rate at which heat is transferred is represented by the symbol q. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt. What Is Q Heat Transfer.
From heattransferkarikuse.blogspot.com
Heat Transfer Heat Transfer Q Equation What Is Q Heat Transfer Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Sometimes it is important to. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. When we want to know the change in enthalpy.. What Is Q Heat Transfer.
From www.simscale.com
What Is Heat Transfer (Heat Flow)? Complete Guide SimScale What Is Q Heat Transfer The rate at which heat is transferred is represented by the symbol q. When we want to know the change in enthalpy. The symbol c stands for the specific heat (also called “. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. What Is Q Heat Transfer.
From www.slideserve.com
PPT SECTION 1 HEAT TRANSFER ANALYSIS PowerPoint Presentation, free What Is Q Heat Transfer The symbol c stands for the specific heat (also called “. \[q=m c \delta t, \nonumber \] where \(q\) is the. The quantitative relationship between heat transfer and temperature change contains all three factors: Sometimes it is important to. The rate at which heat is transferred is represented by the symbol q. Heat is an energy transfer, whereas enthalpy is. What Is Q Heat Transfer.
From www.youtube.com
Heat Transfer L2 p2 Convection Rate Equation Newton's Law of What Is Q Heat Transfer Sometimes it is important to. \[q=m c \delta t, \nonumber \] where \(q\) is the. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. The quantitative relationship between heat transfer and temperature change contains all three factors: When. What Is Q Heat Transfer.
From www.slideshare.net
11 Heat Transfer What Is Q Heat Transfer Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. \[q=m c \delta t, \nonumber \] where \(q\) is the. Heat is transferred between two atoms or molecules in direct contact. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. Q = mcδt, where q is. What Is Q Heat Transfer.
From www.studyiq.com
Heat Transfer Types, Definition, Convection, Radiation, Conduction What Is Q Heat Transfer Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Heat is transferred between two atoms or molecules in direct contact. Sometimes it is important to. Heat transfer is the movement of heat due to a temperature difference between a system and. What Is Q Heat Transfer.
From heattransferkarikuse.blogspot.com
Heat Transfer Heat Transfer Q Equation What Is Q Heat Transfer The symbol c stands for the specific heat (also called “. Heat is transferred between two atoms or molecules in direct contact. Common units for heat ˙q transfer rate is btu/hr. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Heat. What Is Q Heat Transfer.
From spectacularsci.com
What Are The 3 Types of Heat Transfer? Spectacular Science What Is Q Heat Transfer Heat is transferred between two atoms or molecules in direct contact. The rate at which heat is transferred is represented by the symbol q. The quantitative relationship between heat transfer and temperature change contains all three factors: Common units for heat ˙q transfer rate is btu/hr. Sometimes it is important to. The symbol c stands for the specific heat (also. What Is Q Heat Transfer.
From www.youtube.com
Specific Heat Capacity q = mcT Everything you need to know! Chemistry What Is Q Heat Transfer When we want to know the change in enthalpy. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The quantitative relationship between heat transfer and temperature change contains all three factors: Sometimes it is important to. Heat is an energy transfer,. What Is Q Heat Transfer.
From www.youtube.com
HTPIB14B Specific Heat and Q = mcT YouTube What Is Q Heat Transfer Common units for heat ˙q transfer rate is btu/hr. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The quantitative relationship between heat transfer and. What Is Q Heat Transfer.
From www.researchgate.net
Rate of heat transfer q transfer versus rate of mass flow q leak at What Is Q Heat Transfer Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The symbol c stands for the specific heat (also called “. Common units for heat ˙q transfer rate is btu/hr. Heat is transferred between two atoms or molecules in direct contact. The rate at which heat is transferred is represented by the. What Is Q Heat Transfer.
From www.youtube.com
Heat Transfer Chapter 3 Extended Surfaces (Fins) YouTube What Is Q Heat Transfer When we want to know the change in enthalpy. Sometimes it is important to. Common units for heat ˙q transfer rate is btu/hr. The quantitative relationship between heat transfer and temperature change contains all three factors: Heat is transferred between two atoms or molecules in direct contact. The transfer occurs when agitated molecules at high temperatures strike slower molecules at. What Is Q Heat Transfer.
From studylib.net
Heat Equation What Is Q Heat Transfer When we want to know the change in enthalpy. The energy transfer is always from. Heat is transferred between two atoms or molecules in direct contact. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. Sometimes it is important to. The rate at which heat is transferred is represented by the symbol q. Q. What Is Q Heat Transfer.
From www.grc.nasa.gov
Heat Transfer What Is Q Heat Transfer \[q=m c \delta t, \nonumber \] where \(q\) is the. When we want to know the change in enthalpy. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. The quantitative relationship between heat transfer and temperature change contains all three factors:. What Is Q Heat Transfer.
From slidetodoc.com
Heat q Heat the transfer of energy between What Is Q Heat Transfer Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Sometimes it is important to. Common units for heat ˙q transfer rate is btu/hr. \[q=m c \delta t, \nonumber \] where \(q\) is the. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass. What Is Q Heat Transfer.
From www.gbu-presnenskij.ru
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF What Is Q Heat Transfer Common units for heat ˙q transfer rate is btu/hr. The symbol c stands for the specific heat (also called “. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. When we want to know the change in enthalpy. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,.. What Is Q Heat Transfer.
From www.slideserve.com
PPT Heat Transfer Coefficient PowerPoint Presentation, free download What Is Q Heat Transfer Common units for heat ˙q transfer rate is btu/hr. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. The symbol c stands for the specific heat (also called “. The rate at which heat is transferred is represented by the symbol q. Heat is transferred between two atoms or molecules in direct contact. Heat. What Is Q Heat Transfer.
From www.youtube.com
Thermodynamics (Physics) Lesson 2 Heat Transfer and Specific Heat.avi What Is Q Heat Transfer Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Common units for heat ˙q transfer rate is btu/hr. Sometimes it is important to. Heat is transferred between two atoms or molecules in direct contact. When we want to know the change. What Is Q Heat Transfer.
From mungfali.com
Heat Transfer Coefficient Equation What Is Q Heat Transfer The symbol c stands for the specific heat (also called “. \[q=m c \delta t, \nonumber \] where \(q\) is the. Heat is transferred between two atoms or molecules in direct contact. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. Sometimes it is important to. The quantitative relationship between heat transfer and. What Is Q Heat Transfer.
From www.numerade.com
SOLVED P5.16 Convection heat transfer data are often reported as a What Is Q Heat Transfer When we want to know the change in enthalpy. Heat is transferred between two atoms or molecules in direct contact. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in temperature. Common units for heat ˙q transfer rate is btu/hr. The transfer occurs. What Is Q Heat Transfer.
From www.slideserve.com
PPT Overall Heat Transfer Coefficient PowerPoint Presentation, free What Is Q Heat Transfer The energy transfer is always from. Heat is transferred between two atoms or molecules in direct contact. \[q=m c \delta t, \nonumber \] where \(q\) is the. Common units for heat ˙q transfer rate is btu/hr. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a sample. The rate at which heat is transferred is. What Is Q Heat Transfer.
From www.youtube.com
Calculating Rate of Heat Transfer Between Two Working Fluids of a Heat What Is Q Heat Transfer Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. The quantitative relationship between heat transfer and temperature change contains all three factors: Common units for heat ˙q transfer rate is btu/hr. Sometimes it is important to.. What Is Q Heat Transfer.
From www.chegg.com
Solved Units Symbol Q Quantity rate of heat transfer per What Is Q Heat Transfer \[q=m c \delta t, \nonumber \] where \(q\) is the. Heat transfer is the movement of heat due to a temperature difference between a system and its surroundings. Sometimes it is important to. When we want to know the change in enthalpy. The energy transfer is always from. The rate at which heat is transferred is represented by the symbol. What Is Q Heat Transfer.
From me-mechanicalengineering.com
Modes of Heat Transfer What Is Q Heat Transfer The energy transfer is always from. The transfer occurs when agitated molecules at high temperatures strike slower molecules at low temperatures,. Heat is transferred between two atoms or molecules in direct contact. The rate at which heat is transferred is represented by the symbol q. Heat is an energy transfer, whereas enthalpy is a kind of energy content of a. What Is Q Heat Transfer.