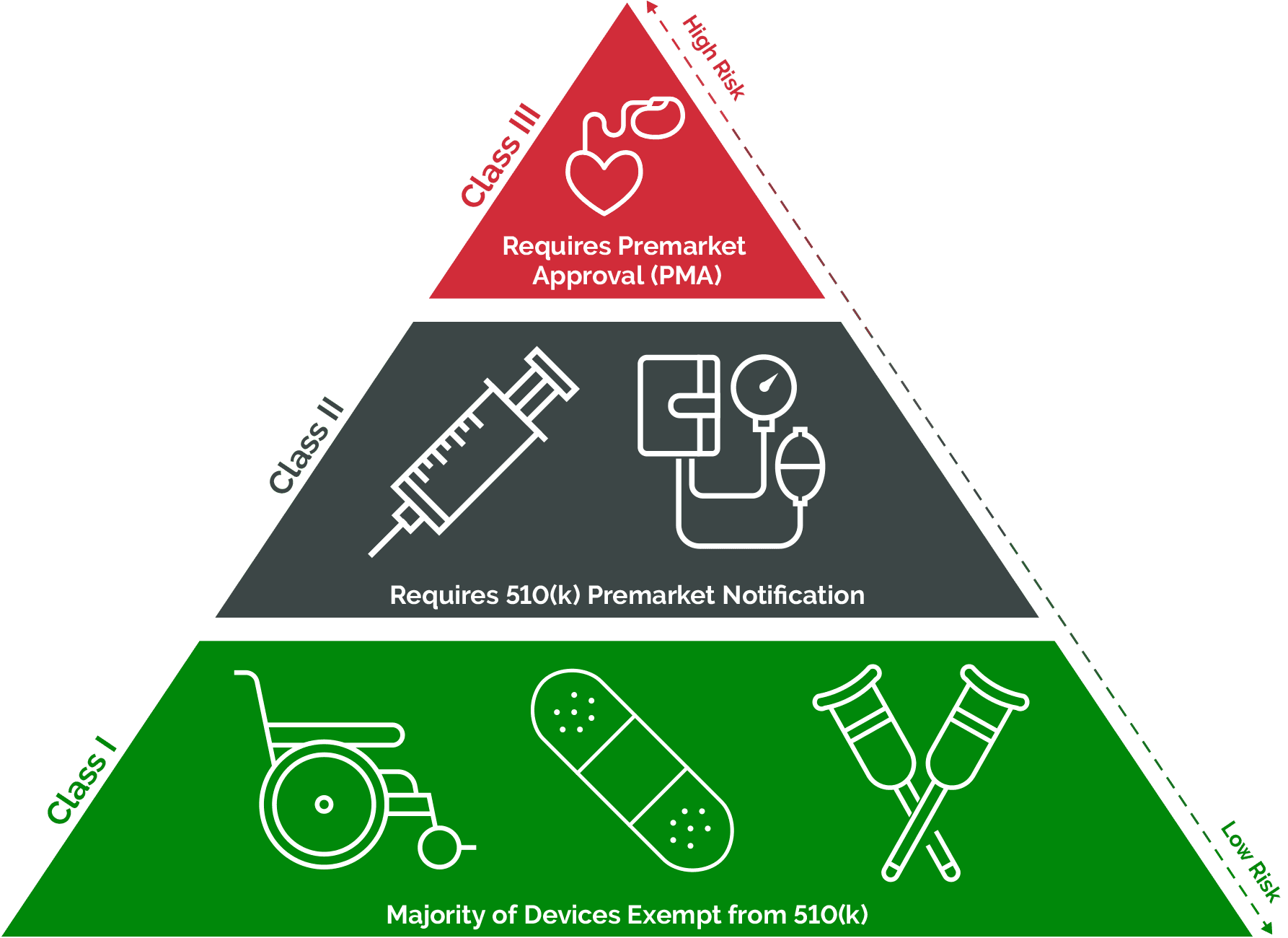
Medical Device Definition As Per Cdsco . (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. 89 rows medical device & diagnostics. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,.
from giodpatut.blob.core.windows.net
(r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. 89 rows medical device & diagnostics. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017.
Medical Device System Definition at Danny Henke blog
Medical Device Definition As Per Cdsco In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. 89 rows medical device & diagnostics.
From pharmadocx.com
Understanding CDSCO's Role in Medical Device Regulation in India Medical Device Definition As Per Cdsco 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks.. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT The Complete Guide to CDSCO Medical Device Registration RSI Medical Device Definition As Per Cdsco Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the. Medical Device Definition As Per Cdsco.
From cdsco.gov.in
Medical device & diagnostics Medical Device Definition As Per Cdsco (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. In. Medical Device Definition As Per Cdsco.
From kantify.com
Kantify Improving Human Health through Artificial Intelligence Medical Device Definition As Per Cdsco The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. Medical devices listed. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT CDSCO Medical Device Grouping Guidelines India RSI PowerPoint Medical Device Definition As Per Cdsco Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO Manufacturing License Get Manufacturing License for Medical Medical Device Definition As Per Cdsco The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. (r) “custom made. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT The Complete Guide to CDSCO Medical Device Registration RSI Medical Device Definition As Per Cdsco In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. (r) “custom made. Medical Device Definition As Per Cdsco.
From vocal.media
CDSCO Classifications of medical devices. 01 Medical Device Definition As Per Cdsco Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and. Medical Device Definition As Per Cdsco.
From www.bizadvisors.io
CDSCO Registration Documents, Benefits, Procedure BizAdvisors Medical Device Definition As Per Cdsco In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO Manufacturing License Get Manufacturing License for Medical Medical Device Definition As Per Cdsco 89 rows medical device & diagnostics. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),.. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT How do you register your medical device with CDSCO? Regulatory Medical Device Definition As Per Cdsco The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. Medical devices listed. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO Classification for medical devices Operon Strategist Medical Device Definition As Per Cdsco (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and. Medical Device Definition As Per Cdsco.
From giodpatut.blob.core.windows.net
Medical Device System Definition at Danny Henke blog Medical Device Definition As Per Cdsco Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription. Medical Device Definition As Per Cdsco.
From pharmadocx.com
Understanding CDSCO's Role in Medical Device Regulation in India Medical Device Definition As Per Cdsco 89 rows medical device & diagnostics. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. In india, all medical devices are regulated under the drugs & cosmetics. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT How do you register your medical device with CDSCO? Regulatory Medical Device Definition As Per Cdsco 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. As per the. Medical Device Definition As Per Cdsco.
From www.linkedin.com
CDSCO Medical Device Manufacturing License, Registration Process, MD5, 9 Medical Device Definition As Per Cdsco As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. 89 rows medical device & diagnostics. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT How do you register your medical device with CDSCO? Regulatory Medical Device Definition As Per Cdsco As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. Medical devices. Medical Device Definition As Per Cdsco.
From operonstrategist.com
Navigating CDSCO Registration for Software as Medical Device Latest Medical Device Definition As Per Cdsco In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO India Medical Device Registration (Documents, Process) IVD Medical Device Definition As Per Cdsco As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. Regulating medical devices poses a real. Medical Device Definition As Per Cdsco.
From us.operonstrategist.com
CDSCO Medical Device Registration Consultants Expert License Medical Device Definition As Per Cdsco In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered. Medical Device Definition As Per Cdsco.
From www.jrcompliance.com
CDSCO Registration Medical Device Registration Certificate Medical Device Definition As Per Cdsco (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. Medical. Medical Device Definition As Per Cdsco.
From www.youtube.com
Importance of CDSCO Certificate In Public Health Domain CDSCO Medical Device Definition As Per Cdsco 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. 89. Medical Device Definition As Per Cdsco.
From www.operonstrategist.com
CDSCO Guideline for Medical Devices Safety Drugs and Cosmetics Act Medical Device Definition As Per Cdsco 89 rows medical device & diagnostics. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. As per the medical device definition the substances including mechanical contraceptives. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT The Complete Guide to CDSCO Medical Device Registration RSI Medical Device Definition As Per Cdsco 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. As. Medical Device Definition As Per Cdsco.
From exoyhcqns.blob.core.windows.net
Medical Device Definition Us Fda at James Grist blog Medical Device Definition As Per Cdsco As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. 89 rows medical device. Medical Device Definition As Per Cdsco.
From omcmedical.com
CDSCO Registration Process OMC Medical Medical Device Definition As Per Cdsco 89 rows medical device & diagnostics. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. The classification of medical devices rules along with regulatory approval and registration by the. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO Medical Devices Registration in India CDSCO MD Online IVD Medical Device Definition As Per Cdsco In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. 89 rows medical device & diagnostics. (r) “custom made medical device” means a medical device made specifically. Medical Device Definition As Per Cdsco.
From cliniexperts.com
Classification Of NonNotified Medical Devices By CDSCO CliniExperts Medical Device Definition As Per Cdsco Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. 89 rows medical device & diagnostics. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO Registration & Certificate CDSCO Medical Device Consultant Medical Device Definition As Per Cdsco In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. 648( e ) dated. Medical Device Definition As Per Cdsco.
From www.corpseed.com
Classification of Medical Devices by CDSCO in India Corpseed Medical Device Definition As Per Cdsco (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. 89 rows medical device & diagnostics. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical. Medical Device Definition As Per Cdsco.
From pharmadocx.com
CDSCO Import License Consultant for Medical Devices in India Medical Device Definition As Per Cdsco As per the medical device definition the substances including mechanical contraceptives (condoms, intrauterine devices, tubal rings),. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. The classification of medical. Medical Device Definition As Per Cdsco.
From www.slideserve.com
PPT How do you register your medical device with CDSCO? Regulatory Medical Device Definition As Per Cdsco 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which ultimately aim at replacing the. Medical. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO Notification For Class A Nonsterile And Non Measuring Medical Medical Device Definition As Per Cdsco Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on associated risks. In india, all medical devices are regulated under the drugs & cosmetics act, 1940 and medical devices rules, 2017. The classification of medical devices rules along with regulatory approval and registration by the cdsco is under. Medical Device Definition As Per Cdsco.
From operonstrategist.com
CDSCO India Medical Device Registration (Documents, Process) IVD Medical Device Definition As Per Cdsco 89 rows medical device & diagnostics. (r) “custom made medical device” means a medical device made specifically in accordance with a written prescription of a registered medical. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. Regulating medical devices poses a real challenge, upon implementation of the medical device rules, 2017 which. Medical Device Definition As Per Cdsco.
From gioetfubc.blob.core.windows.net
Device Class Definition at Daniel Hart blog Medical Device Definition As Per Cdsco The classification of medical devices rules along with regulatory approval and registration by the cdsco is under the control of drug. 648( e ) dated 11.02.2020 all devices including an instrument, apparatus, appliance, implant, material or other article,. Medical devices listed under the new rules “medical devices rules, 2017” are categorized as per the global harmonization task force depending on. Medical Device Definition As Per Cdsco.