Pink Eye Drops Recall . These products may cause serious harm to users. The pendopharm division of pharmascience inc. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. The pendopharm division of pharmascience inc. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due.
from cristinwbinni.pages.dev
The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The pendopharm division of pharmascience inc. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. These products may cause serious harm to users. The pendopharm division of pharmascience inc. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due.
List Of Eye Drops Recall 2024 Raine Carolina
Pink Eye Drops Recall The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The pendopharm division of pharmascience inc. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The pendopharm division of pharmascience inc. These products may cause serious harm to users. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk.
From kareeqmurial.pages.dev
List Of Eye Drops Recall 2025 Betta Charlot Pink Eye Drops Recall The pendopharm division of pharmascience inc. The pendopharm division of pharmascience inc. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. These products may cause serious harm to. Pink Eye Drops Recall.
From www.aarp.org
2 More Eye Drop Brands Recalled Due to Safety Risks Pink Eye Drops Recall The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The fda sent warning letters to several companies for illegally. Pink Eye Drops Recall.
From rosaliawashien.pages.dev
2024 Eye Drop Recall List Bobby Christa Pink Eye Drops Recall The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The pendopharm division of pharmascience inc. The recall is based on unsanitary conditions and bacteria found in the manufacturing. Pink Eye Drops Recall.
From www.entrepreneur.com
Every Eye Drop and Gel Being Recalled From Target, CVS, Rite Aid Pink Eye Drops Recall The pendopharm division of pharmascience inc. These products may cause serious harm to users. The pendopharm division of pharmascience inc. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The. Pink Eye Drops Recall.
From www.kare11.com
Eye drop recall 2023 FDA warns about bacteria contaminated items Pink Eye Drops Recall These products may cause serious harm to users. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial. Pink Eye Drops Recall.
From www.usatoday.com
Eye drop recall stirs concern about overthecounter drug safety Pink Eye Drops Recall The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The pendopharm division of pharmascience inc. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops. Pink Eye Drops Recall.
From cristinwbinni.pages.dev
List Of Eye Drops Recall 2024 Raine Carolina Pink Eye Drops Recall The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. These products may cause serious harm to users. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The pendopharm division of pharmascience inc. The recall is based on unsanitary conditions and. Pink Eye Drops Recall.
From abc13.com
Eye drops recall FDA warns to immediately stop using 26 types of over Pink Eye Drops Recall The pendopharm division of pharmascience inc. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink. Pink Eye Drops Recall.
From www.recallguide.org
Pink Eye Remedy Information, Side Effects, Warnings and Recalls Pink Eye Drops Recall Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. These products may cause serious harm to users. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The pendopharm division of pharmascience inc. The pendopharm division of pharmascience inc. The. Pink Eye Drops Recall.
From www.sportskeeda.com
Eye Drops Recall 2023 Reasons, brand, and all you need to know Pink Eye Drops Recall The pendopharm division of pharmascience inc. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. These products may cause serious harm to users. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. Is recalling all lots of the 10 ml format of. Pink Eye Drops Recall.
From mynews13.com
Recall Walgreens, Walmart Eye Drops May Not be Sterile Pink Eye Drops Recall The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma. Pink Eye Drops Recall.
From www.everydayhealth.com
FDA Recall for Eye Drops From Walmart, Target, CVS, Rite Aid Pink Eye Drops Recall These products may cause serious harm to users. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the. Pink Eye Drops Recall.
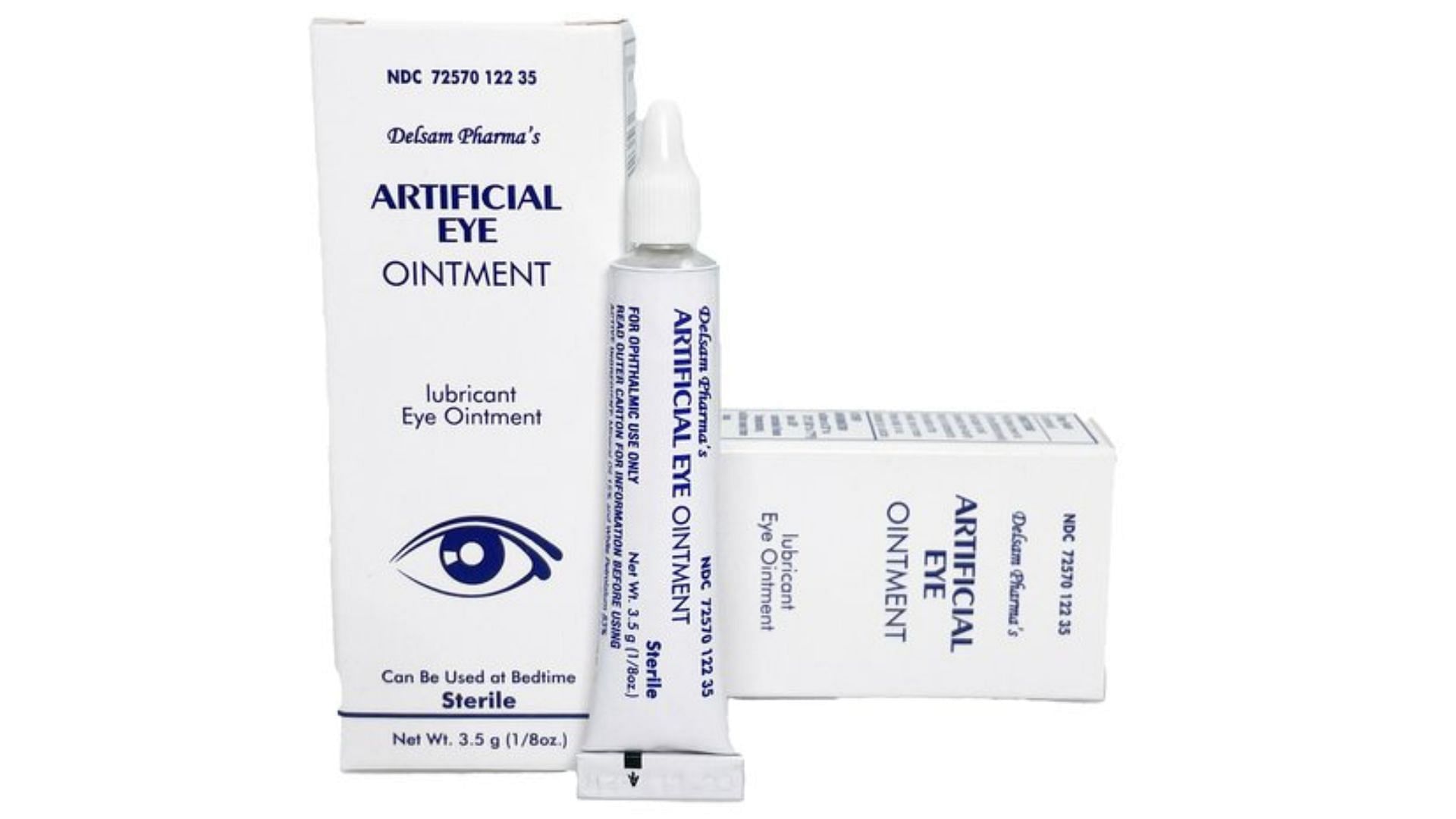
From www.aboutlawsuits.com
FDA Adds Delsam Pharma’s Artificial Eye Ointment to List of Recalled Pink Eye Drops Recall Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The pendopharm division of pharmascience inc. The pendopharm division of pharmascience inc. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The fda has expanded its list of eye drops recalled in. Pink Eye Drops Recall.
From www.msn.com
Eye Drops Recall 2023 Reasons, brand, and all you need to know Pink Eye Drops Recall Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The pendopharm division of pharmascience inc. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The fda sent warning letters to several companies for illegally marketing eye products that claim. Pink Eye Drops Recall.
From depybtfweco.blob.core.windows.net
Eyes Burn After Allergy Eye Drops at Arnold Carrier blog Pink Eye Drops Recall Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The pendopharm division of pharmascience inc. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The. Pink Eye Drops Recall.
From faunqphillida.pages.dev
Are Refresh Eye Drops Recalled October 2024 Alyda Bernita Pink Eye Drops Recall Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The pendopharm division of pharmascience inc. The fda sent warning letters to several companies for illegally marketing eye products that claim. Pink Eye Drops Recall.
From www.cbs8.com
Eye drop recall More deaths, injuries linked to bacteria Pink Eye Drops Recall The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The pendopharm division of pharmascience. Pink Eye Drops Recall.
From prudiamignon.pages.dev
Eye Drop Recall 2024 Cdc Nicki Amabelle Pink Eye Drops Recall These products may cause serious harm to users. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. The recall is based on unsanitary conditions and bacteria found in. Pink Eye Drops Recall.
From abc7news.com
Eye drops recalls Why are so many eye drops being recalled? Amazon Pink Eye Drops Recall The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The pendopharm division of. Pink Eye Drops Recall.
From anneymallorie.pages.dev
Which Eye Drops Have Been Recalled In 2025 Maye Harriet Pink Eye Drops Recall The pendopharm division of pharmascience inc. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The. Pink Eye Drops Recall.
From abc13.com
Eye drops recall FDA warns to immediately stop using 26 types of over Pink Eye Drops Recall The pendopharm division of pharmascience inc. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. These products may cause serious harm to users. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The recall is based on unsanitary conditions. Pink Eye Drops Recall.
From dnyuz.com
Which eye drops have been recalled and why are they dangerous? DNyuz Pink Eye Drops Recall These products may cause serious harm to users. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The pendopharm division of pharmascience inc. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. Is recalling all lots of the 10 ml format of. Pink Eye Drops Recall.
From il.iherb.com
Similasan, Pink Eye Relief, Sterile Eye Drops, 0.33 fl oz (10 ml) iHerb Pink Eye Drops Recall Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The pendopharm division of pharmascience inc. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink. Pink Eye Drops Recall.
From jordainwjeri.pages.dev
Eye Drop Recall 2024 Brands List Susi Zilvia Pink Eye Drops Recall The pendopharm division of pharmascience inc. The pendopharm division of pharmascience inc. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. Is recalling all lots of the 10. Pink Eye Drops Recall.
From amalleqsarena.pages.dev
What Eye Drops Were Recalled 2024 Fiona Jessica Pink Eye Drops Recall The pendopharm division of pharmascience inc. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The pendopharm division of pharmascience inc. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The fda sent warning letters to several companies for illegally marketing eye. Pink Eye Drops Recall.
From www.southernliving.com
Eye Drops Recalled Nationwide Over Contamination Risk Pink Eye Drops Recall Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The pendopharm division of pharmascience inc. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The recall is based on unsanitary conditions and bacteria found in the. Pink Eye Drops Recall.
From www.goodhousekeeping.com
Eye Drops Recall 2024 Full List of Brands, Retailers and More Pink Eye Drops Recall The pendopharm division of pharmascience inc. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The pendopharm division of. Pink Eye Drops Recall.
From alerts.eyedropsafety.org
Are My Eye Drops Safe? Pink Eye Drops Recall The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. These products may cause serious harm to users. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial. Pink Eye Drops Recall.
From dailymed.nlm.nih.gov
DailyMed PINK EYE belladonna, euphrasia officinalis, hepar sulphuris Pink Eye Drops Recall The pendopharm division of pharmascience inc. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. These products may cause serious harm to users. Is recalling all lots of the 5 ml. Pink Eye Drops Recall.
From www.wthr.com
Several eye drops and ointment sold at Walmart recalled for being Pink Eye Drops Recall The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The pendopharm division of pharmascience inc. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. Is recalling all lots of the 10 ml format of cromolyn eye drops. Pink Eye Drops Recall.
From www.sportskeeda.com
Eye Drops Recall 2022 Everything to know about Pharmasave and Pink Eye Drops Recall The fda has expanded its list of eye drops recalled in 2023 due to bacteria contamination and infection risk. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and.. Pink Eye Drops Recall.
From www.washingtonpost.com
More eye drops are being recalled. Here's what to know. The Pink Eye Drops Recall Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of microbial growth. The. Pink Eye Drops Recall.
From www.bloomberg.com
CVS, Walgreens Warned Over Eyedrops After Deadly Bacterial Outbreak Pink Eye Drops Recall The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The pendopharm division of pharmascience inc. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. Is recalling all lots of the 10 ml format of cromolyn eye drops due to the risk of. Pink Eye Drops Recall.
From www.aboutlawsuits.com
Lubrisine Eye Drops Recall Sterility Concerns And Undeclared Pink Eye Drops Recall Is recalling all lots of the 5 ml and 10 ml formats of cromolyn eye drops due. These products may cause serious harm to users. The pendopharm division of pharmascience inc. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The fda sent warning letters to several companies for illegally. Pink Eye Drops Recall.
From ndclist.com
FDA Recall Pink Eye Relief Liquid Ophthalmic Pink Eye Drops Recall The recall is based on unsanitary conditions and bacteria found in the manufacturing facility. The agency said the products are illegally marketed to treat conditions including conjunctivitis — pink eye — glaucoma and. The fda sent warning letters to several companies for illegally marketing eye products that claim to treat cataracts, glaucoma and other conditions. Is recalling all lots of. Pink Eye Drops Recall.