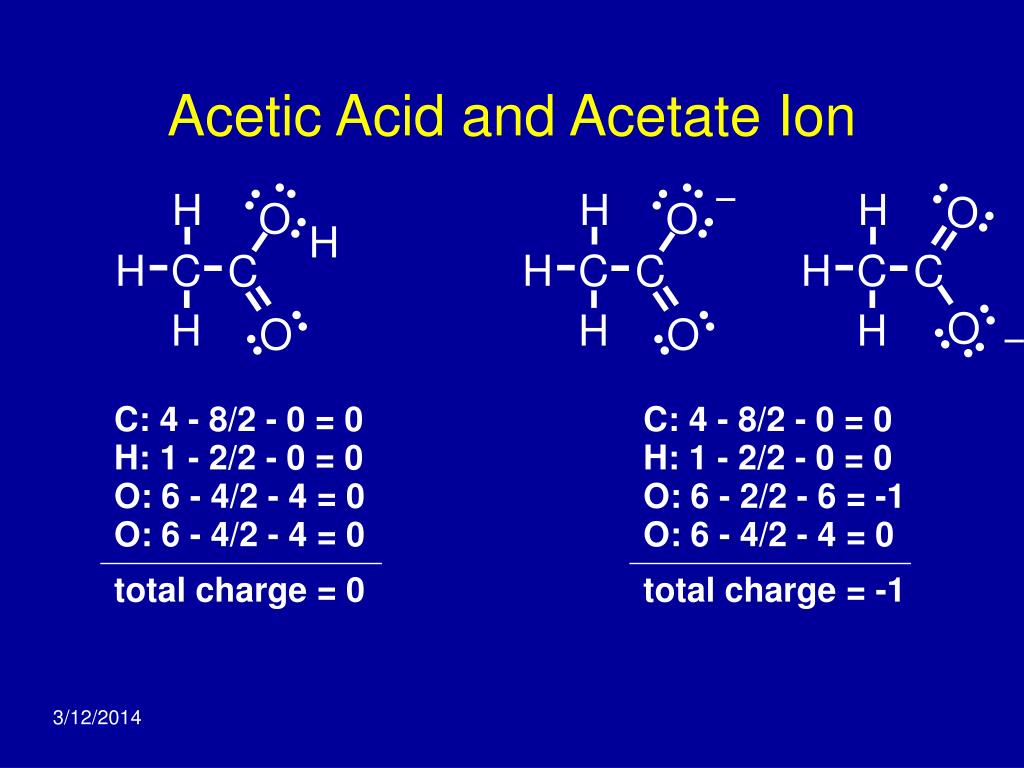
Acetic Acid Charge . Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. C 2 h 4 o 2. For acetic acid, however, there is a key difference: Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols.
from ar.inspiredpencil.com
For acetic acid, however, there is a key difference: The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. C 2 h 4 o 2. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid.
Acetic Acid Lewis Structure With Formal Charges
Acetic Acid Charge Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. For acetic acid, however, there is a key difference: Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. C 2 h 4 o 2.
From stock.adobe.com
Molecular structure of acetic acid, 3d rendering Stock Illustration Acetic Acid Charge Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Two resonance contributors can be drawn for the. Acetic Acid Charge.
From www.alamy.com
Acetic Acid, Structural chemical formula on a white background Stock Acetic Acid Charge C 2 h 4 o 2. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Learn about the geometry,. Acetic Acid Charge.
From www.seastarchemicals.com
Acetic Acid SEASTAR CHEMICALS Acetic Acid Charge C 2 h 4 o 2. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. For acetic acid,. Acetic Acid Charge.
From mungfali.com
Chemical Structure Of Acetic Acid Acetic Acid Charge Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. For acetic acid, however, there is a key difference: C 2 h 4 o 2. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. The main distinction between acetate and acetic acid is that acetic acid is a neutral. Acetic Acid Charge.
From www.alamy.com
Acetic acid hires stock photography and images Alamy Acetic Acid Charge Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids. Acetic Acid Charge.
From www.istockphoto.com
Vetores de Molécula De Ácido Acético e mais imagens de Bioquímica Acetic Acid Charge For acetic acid, however, there is a key difference: Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. C 2 h 4 o 2. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Acetic acid is an organic compound that helps create vinegar while acetate. Acetic Acid Charge.
From www.bio-world.com
Acetic Acid Glacial Acids and Bases bioWORLD Acetic Acid Charge Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. C 2 h 4 o 2. Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Acetic acid is an organic compound that. Acetic Acid Charge.
From ar.inspiredpencil.com
Lewis Structure Of Acetic Acid And Most Acidic Proton Acetic Acid Charge For acetic acid, however, there is a key difference: Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion. Acetic Acid Charge.
From www.sciencephoto.com
Acetic acid molecule, illustration Stock Image F027/8027 Science Acetic Acid Charge The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. For acetic acid, however, there is a key difference: Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Learn about the geometry, electronic. Acetic Acid Charge.
From collegedunia.com
Uses of Acetic Acid Definition, Structure, and Physical and Chemical Acetic Acid Charge Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Learn about the. Acetic Acid Charge.
From www.dreamstime.com
Acetic Acid Hand Drawn Vector Formula Chemical Structure Lettering Blue Acetic Acid Charge C 2 h 4 o 2. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Two resonance contributors can be drawn for the conjugate base,. Acetic Acid Charge.
From wiki.opensourceecology.org
Acetic Acid Open Source Ecology Acetic Acid Charge Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main distinction between acetate and acetic acid. Acetic Acid Charge.
From www.vectorstock.com
Formula and model of acetic acid molecule Vector Image Acetic Acid Charge Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. For acetic acid, however, there is a key difference: C 2 h 4 o 2. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Find out how resonance, conjugation and pka values affect the. Acetic Acid Charge.
From www.youtube.com
CH3COOH Lewis Structure (Acetic acid) YouTube Acetic Acid Charge C 2 h 4 o 2. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Two resonance. Acetic Acid Charge.
From www.vectorstock.com
C2h4o2 acetic acid molecule Royalty Free Vector Image Acetic Acid Charge Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s. Acetic Acid Charge.
From ar.inspiredpencil.com
Acetic Acid Lewis Structure With Formal Charges Acetic Acid Charge For acetic acid, however, there is a key difference: Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. C 2 h 4 o 2. Find out. Acetic Acid Charge.
From www.glentham.com
Acetic acid (CAS 64197) Glentham Life Sciences Acetic Acid Charge Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main distinction between acetate and acetic acid is that acetic acid is a. Acetic Acid Charge.
From www.alamy.com
Acetic acid molecule hires stock photography and images Alamy Acetic Acid Charge Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. C. Acetic Acid Charge.
From www.dreamstime.com
Acetic Acid, Structural Chemical Formula Stock Illustration Acetic Acid Charge Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s. Acetic Acid Charge.
From mungfali.com
Chemical Structure Of Acetic Acid Acetic Acid Charge For acetic acid, however, there is a key difference: C 2 h 4 o 2. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Two resonance contributors can be drawn for the conjugate base, and the. Acetic Acid Charge.
From cymitquimica.com
Acetic Acid CymitQuimica Acetic Acid Charge Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. C 2 h 4 o 2. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net. Acetic Acid Charge.
From www.alamy.com
Acetic acid (ethanoic acid) organic chemistry lesson Stock Vector Acetic Acid Charge Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. C 2 h 4 o 2. For acetic acid, however, there is a key difference: The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Learn. Acetic Acid Charge.
From www.sciencephoto.com
Acetic acid molecule Stock Image F012/5730 Science Photo Library Acetic Acid Charge Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. For acetic acid, however, there is a key difference: Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main. Acetic Acid Charge.
From quizlet.com
Draw the Lewis structure for acetic acid. Quizlet Acetic Acid Charge C 2 h 4 o 2. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. For acetic acid, however, there is a key difference: Two resonance contributors can be drawn for the conjugate. Acetic Acid Charge.
From commons.wikimedia.org
FileAceticacidCRCGED3DballsA.png Acetic Acid Charge For acetic acid, however, there is a key difference: Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. C 2 h 4. Acetic Acid Charge.
From mavink.com
Acetic Acid Molecular Structure Acetic Acid Charge Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Acetic acid is. Acetic Acid Charge.
From www.alamy.com
Acetic acid molecule, illustration Stock Photo Alamy Acetic Acid Charge Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. C 2 h 4 o 2. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Acetic acid is an organic compound that helps create vinegar. Acetic Acid Charge.
From www.wikidoc.org
Acetic acid wikidoc Acetic Acid Charge The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. Two resonance contributors can be drawn for the conjugate base, and the negative charge. Acetic Acid Charge.
From chemicaldb.netlify.app
How stable is acetic acid Acetic Acid Charge C 2 h 4 o 2. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. For acetic acid, however, there is a key difference: Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. The main distinction between acetate and acetic acid is. Acetic Acid Charge.
From psychology.wikia.com
Acetic acid Psychology Wiki Acetic Acid Charge Acetic acid is an organic compound that helps create vinegar while acetate ion is the acetic acid’s conjugate base. C 2 h 4 o 2. The main distinction between acetate and acetic acid is that acetic acid is a neutral compound, while acetate is an anion with a net negative electric charge. Inductive effects and charge delocalization significantly influence the. Acetic Acid Charge.
From heilprocessequipment.com
Acetic Acid Heil Process Equipment Acetic Acid Charge For acetic acid, however, there is a key difference: Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Acetic acid. Acetic Acid Charge.
From structure-of.blogspot.com
Acetic acid Structure of Acetic Acid Charge Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. For acetic acid, however, there is a key difference: C 2 h 4 o 2. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Acetic acid is an organic compound that helps create vinegar while acetate ion. Acetic Acid Charge.
From www.sciencephoto.com
Acetic acid molecule, illustration Stock Image F027/8025 Science Acetic Acid Charge Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. C 2 h 4 o 2. For acetic acid, however, there is a key difference: Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. Inductive effects and charge delocalization significantly influence the acidity or basicity of. Acetic Acid Charge.
From fr.wikidoc.org
Acetic acid wikidoc Acetic Acid Charge C 2 h 4 o 2. Two resonance contributors can be drawn for the conjugate base, and the negative charge can be. Inductive effects and charge delocalization significantly influence the acidity or basicity of a compound. For acetic acid, however, there is a key difference: The main distinction between acetate and acetic acid is that acetic acid is a neutral. Acetic Acid Charge.
From www.dreamstime.com
Acetic Acid, Structural Chemical Formula Stock Photo Image of Acetic Acid Charge Learn about the geometry, electronic structure and hydrogen bonding of carboxylic acids, such as acetic acid. For acetic acid, however, there is a key difference: Find out how resonance, conjugation and pka values affect the acidity of carboxylic acids compared to alcohols. C 2 h 4 o 2. Two resonance contributors can be drawn for the conjugate base, and the. Acetic Acid Charge.