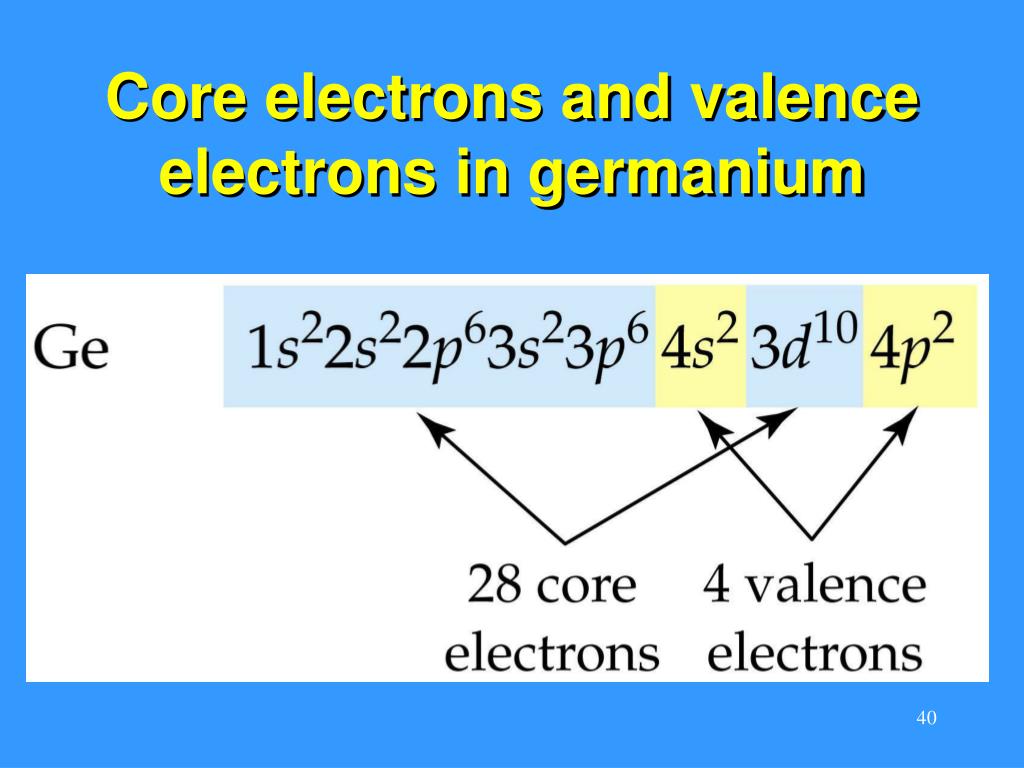
Core Electrons . Core electrons are the electrons in an atom that are not involved in chemical bonding. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. They reside in the inner shells of an atom, closer to the.
from enginedbfascinator.z22.web.core.windows.net
The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The noble gas notation represents many core electrons which are not being shown. Core electrons are the electrons in an atom that are not involved in chemical bonding. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration.
Germanium Ground State Electron Configuration
Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. They reside in the inner shells of an atom, closer to the. Core electrons are the electrons in an atom that are not involved in chemical bonding. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration.
From piratarmy.com
Effective Nuclear Charge Of Oxygen Core Electrons Core electrons are the electrons in an atom that are not involved in chemical bonding. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. The noble gas notation represents many core electrons which are not being shown. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding,. Core Electrons.
From kaylumnegan.blogspot.com
36+ electronic configuration calculator KaylumNegan Core Electrons Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. The noble gas notation represents many core electrons which are not being shown. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn the difference between valence and core electrons, how they. Core Electrons.
From www.slideserve.com
PPT Key Terms Chapter 7 PowerPoint Presentation, free download ID Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. Core electrons are the electrons in an atom that are not involved in chemical bonding. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons. Core Electrons.
From www.chegg.com
Solved Ne 1 SO p Neon has core electrons in the n= level and Core Electrons Core electrons are the electrons in an atom that are not involved in chemical bonding. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. They reside in the inner shells of an atom, closer to the. Learn how to identify valence electrons and core electrons using the periodic. Core Electrons.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID Core Electrons They reside in the inner shells of an atom, closer to the. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble. Core Electrons.
From enginedbfascinator.z22.web.core.windows.net
Germanium Ground State Electron Configuration Core Electrons Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Core electrons are the electrons in an atom that are not involved in chemical bonding. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn the difference between valence and core electrons,. Core Electrons.
From www.slideserve.com
PPT Xray Photoelectron Spectroscopy PowerPoint Presentation, free Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. They reside in the inner shells of an atom, closer to the. Core electrons are the. Core Electrons.
From www.numerade.com
SOLVEDWrite full electron configurations and indicate the valence Core Electrons The noble gas notation represents many core electrons which are not being shown. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. They reside in the inner shells of an atom, closer to the. The electrons occupying the outermost shell orbital(s) (highest value of n) are. Core Electrons.
From slideplayer.com
Valence and Core Electrons ppt download Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. They reside in the inner shells of an atom, closer to the. Core electrons are the. Core Electrons.
From www.pearson.com
How many core electrons does the gallium atom possess? Pearson+ Channels Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. They reside in the inner shells of an atom, closer to the. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons using the periodic. Core Electrons.
From energyeducation.ca
Valence and core electrons Energy Education Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Core electrons are the electrons in an atom that are not involved in chemical bonding. The noble gas notation represents many. Core Electrons.
From slideplayer.com
ELECTRON CONFIGURATION. ppt download Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. They reside in the inner shells of an atom, closer to the. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Core electrons are the electrons in an atom that. Core Electrons.
From rosstable.blogspot.co.uk
The Ross Periodic Table Core Charge Its Periodicity Across the Table Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Core electrons are the electrons in an atom that are not involved in chemical bonding. They reside in the inner shells of an atom, closer to the. Learn how to identify valence electrons and core electrons using the periodic. Core Electrons.
From www.slideserve.com
PPT Core Electrons PowerPoint Presentation, free download ID6788046 Core Electrons They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. Core electrons are the electrons in an atom that are not involved in chemical bonding. The electrons occupying the outermost shell orbital(s) (highest value of. Core Electrons.
From www.slideserve.com
PPT Valence and Core Electrons PowerPoint Presentation, free download Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. They reside in the inner shells of an atom, closer to the. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons using the periodic table and. Core Electrons.
From www.youtube.com
Valence Electrons Explained and Shortcuts on the Periodic Table YouTube Core Electrons They reside in the inner shells of an atom, closer to the. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. The noble gas notation represents many core electrons which are not. Core Electrons.
From www.slideserve.com
PPT CH 8 Electron Configuration & periodicity PowerPoint Core Electrons They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons using the periodic. Core Electrons.
From goodttorials.blogspot.com
How To Find Core Electrons Of An Element Core Electrons They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn how to identify. Core Electrons.
From linksofstrathaven.com
How Many Valence Electrons Does Te Have? Update New Core Electrons Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The noble gas notation represents many core electrons which are not being shown. The electrons occupying the outermost shell orbital(s) (highest. Core Electrons.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. They reside in the inner shells of an atom, closer to the. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Core electrons are the electrons in an atom that are not. Core Electrons.
From jamaicahomebuilders.blogspot.com
Number Of Valence Electrons Definition / Difference Between Valency and Core Electrons Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Core electrons are the electrons in an atom that are not involved in chemical bonding. They reside in the inner shells of an atom, closer to the. The noble gas notation represents many core electrons which are not being shown. The electrons occupying the. Core Electrons.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. Core electrons are the electrons in an atom that are not involved in chemical bonding. They reside in the inner shells. Core Electrons.
From www.slideserve.com
PPT Lecture IX Crystals dr hab. Ewa Popko PowerPoint Presentation Core Electrons Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert.. Core Electrons.
From isiah-blogmahoney.blogspot.com
How Many Valence Electrons Are in an Atom of Sodium Core Electrons Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. The noble gas notation represents many core electrons which are not being shown. Learn the difference between valence and core electrons, how they. Core Electrons.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo Core Electrons Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration.. Core Electrons.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Core Electrons Core electrons are the electrons in an atom that are not involved in chemical bonding. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and. Core Electrons.
From facts.net
14 Intriguing Facts About Valence Electron Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate. Core Electrons.
From www.dreamstime.com
Atom of Scandium with Core and 21 Electrons on White Stock Illustration Core Electrons They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The noble gas notation represents many core electrons which are not being shown. Core electrons are the electrons in an atom that are not involved. Core Electrons.
From www.pinterest.co.kr
The UVS atomic model with a nested dualcore electron shell. Electrons Core Electrons Core electrons are the electrons in an atom that are not involved in chemical bonding. The noble gas notation represents many core electrons which are not being shown. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. They reside in the inner shells of an atom, closer to the. Learn the difference between. Core Electrons.
From ar.inspiredpencil.com
Electron Chemistry Core Electrons Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. The noble gas notation represents many core electrons which are not being shown. Core electrons are the electrons in an atom that are not involved in chemical bonding. They reside in the inner shells of an atom, closer to the. The electrons occupying the. Core Electrons.
From www.chegg.com
Solved Draw the molecular orbital (MO) electron diagram for Core Electrons They reside in the inner shells of an atom, closer to the. Core electrons are the electrons in an atom that are not involved in chemical bonding. The noble gas notation represents many core electrons which are not being shown. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases. Core Electrons.
From slideplayer.com
Valence and Core Electrons ppt download Core Electrons They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn how to identify. Core Electrons.
From www.chegg.com
Solved + Draw the molecular orbital (MO) electron diagram Core Electrons The noble gas notation represents many core electrons which are not being shown. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn the difference between valence and core electrons, how they participate in chemical reactions and bonding, and why noble gases are chemically inert. They reside in. Core Electrons.
From www.chegg.com
Solved Ar core electrons Ar valence electrons Be core Core Electrons The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn how to identify valence electrons and core electrons using the periodic table and electron configuration. They reside in the inner shells of an atom, closer to the. Learn the difference between valence and core electrons, how they participate. Core Electrons.
From www.youtube.com
CHEMISTRY 101 Valence and core electrons YouTube Core Electrons They reside in the inner shells of an atom, closer to the. Core electrons are the electrons in an atom that are not involved in chemical bonding. The electrons occupying the outermost shell orbital(s) (highest value of n) are called valence electrons, and those occupying the inner shell. Learn how to identify valence electrons and core electrons using the periodic. Core Electrons.