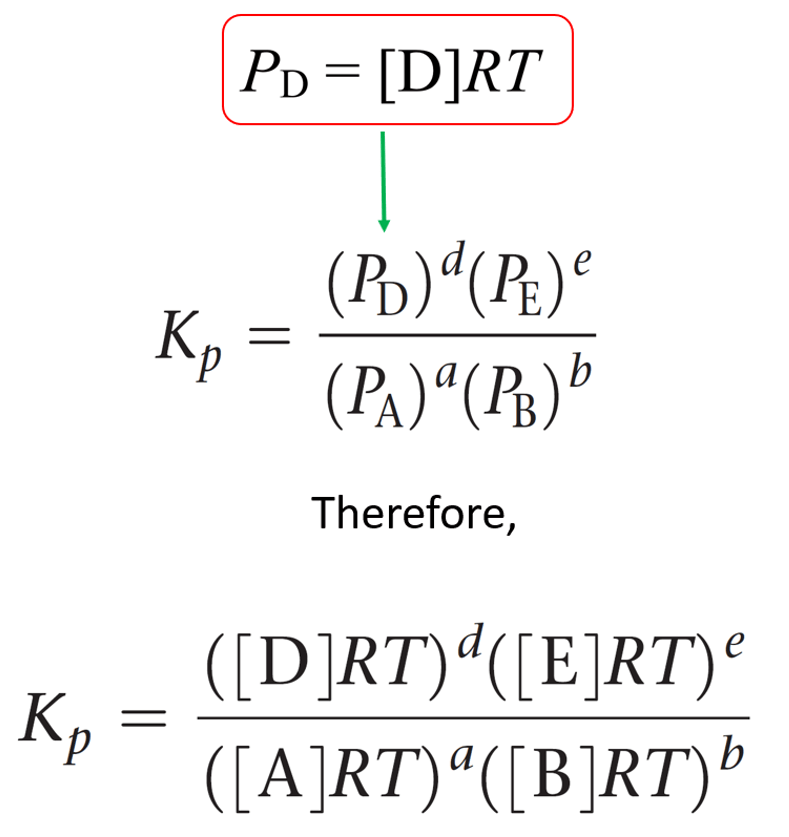What Is Equilibrium Constant Kp . this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. K p is equilibrium constant used when equilibrium. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. It covers an explanation of the. When a reaction is at equilibrium, the forward and reverse reaction rate are. the equilibrium constant, k, is a number that describes the relationship between the number of products and. for a general reaction of gases, the equilibrium constant kp can be represented as: Let’s see an example of calculating. k p and k c are the equilibrium constant of an ideal gaseous mixture. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. Aa + bb ⇆ cc + dd. equilibrium constant kp definition.
from general.chemistrysteps.com
Let’s see an example of calculating. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. Aa + bb ⇆ cc + dd. the equilibrium constant, k, is a number that describes the relationship between the number of products and. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. When a reaction is at equilibrium, the forward and reverse reaction rate are. k p and k c are the equilibrium constant of an ideal gaseous mixture. equilibrium constant kp definition. K p is equilibrium constant used when equilibrium. for a general reaction of gases, the equilibrium constant kp can be represented as:
Kp Equilibrium Constant and Partial Pressure Chemistry Steps
What Is Equilibrium Constant Kp It covers an explanation of the. the equilibrium constant, k, is a number that describes the relationship between the number of products and. Aa + bb ⇆ cc + dd. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. K p is equilibrium constant used when equilibrium. Let’s see an example of calculating. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. It covers an explanation of the. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. k p and k c are the equilibrium constant of an ideal gaseous mixture. for a general reaction of gases, the equilibrium constant kp can be represented as: equilibrium constant kp definition. When a reaction is at equilibrium, the forward and reverse reaction rate are.
From study.com
How to Calculate an Equilibrium Constant Kp Using Partial Pressures What Is Equilibrium Constant Kp When a reaction is at equilibrium, the forward and reverse reaction rate are. k p and k c are the equilibrium constant of an ideal gaseous mixture. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. for a general reaction of gases, the equilibrium. What Is Equilibrium Constant Kp.
From www.youtube.com
Ch 8 Part 4 Equilibrium Constant Kp YouTube What Is Equilibrium Constant Kp the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. It covers an explanation of the. this page explains equilibrium constants expressed in terms of partial pressures. What Is Equilibrium Constant Kp.
From www.chegg.com
Solved The equilibrium constant, Kp, for the following What Is Equilibrium Constant Kp When a reaction is at equilibrium, the forward and reverse reaction rate are. for a general reaction of gases, the equilibrium constant kp can be represented as: an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. K p is equilibrium constant used when equilibrium. Aa. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT The Equilibrium Constant PowerPoint Presentation, free download What Is Equilibrium Constant Kp an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. the equilibrium constant, k, is a number that describes the relationship between the number of products and. When a reaction. What Is Equilibrium Constant Kp.
From study.com
How to Write Pressure Equilibrium Constant Expressions Chemistry What Is Equilibrium Constant Kp Aa + bb ⇆ cc + dd. Let’s see an example of calculating. the equilibrium constant, k, is a number that describes the relationship between the number of products and. When a reaction is at equilibrium, the forward and reverse reaction rate are. It covers an explanation of the. an equilibrium constant calculated from partial pressures (\(k_p\)) is. What Is Equilibrium Constant Kp.
From www.youtube.com
Equilibria Calculating the equilibrium constant Kp. Qu. 2 of 2 YouTube What Is Equilibrium Constant Kp It covers an explanation of the. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. Aa + bb ⇆ cc + dd. Let’s see an example of calculating. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)),. What Is Equilibrium Constant Kp.
From www.youtube.com
How to Write Equilibrium Constant Expression Kc and Kp Expression What Is Equilibrium Constant Kp this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Aa + bb ⇆ cc + dd. equilibrium constant kp definition. Let’s see an example of calculating. k p and k c are the equilibrium constant of an ideal gaseous mixture. It covers an explanation of the. an equilibrium constant calculated. What Is Equilibrium Constant Kp.
From www.slideshare.net
Chapter 15 Lecture Chemical Equilibrium What Is Equilibrium Constant Kp It covers an explanation of the. for a general reaction of gases, the equilibrium constant kp can be represented as: k p and k c are the equilibrium constant of an ideal gaseous mixture. Let’s see an example of calculating. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download What Is Equilibrium Constant Kp It covers an explanation of the. When a reaction is at equilibrium, the forward and reverse reaction rate are. Aa + bb ⇆ cc + dd. the equilibrium constant, k, is a number that describes the relationship between the number of products and. Let’s see an example of calculating. this page explains equilibrium constants expressed in terms of. What Is Equilibrium Constant Kp.
From www.youtube.com
Equilibria Relationship between equilibrium constants Kp & Kc. YouTube What Is Equilibrium Constant Kp K p is equilibrium constant used when equilibrium. Aa + bb ⇆ cc + dd. for a general reaction of gases, the equilibrium constant kp can be represented as: When a reaction is at equilibrium, the forward and reverse reaction rate are. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation What Is Equilibrium Constant Kp k p and k c are the equilibrium constant of an ideal gaseous mixture. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. When a reaction is at equilibrium, the forward and reverse reaction rate are. K p is equilibrium constant used when equilibrium. the equilibrium constant, k,. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download What Is Equilibrium Constant Kp for a general reaction of gases, the equilibrium constant kp can be represented as: Aa + bb ⇆ cc + dd. When a reaction is at equilibrium, the forward and reverse reaction rate are. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. It covers an explanation of the.. What Is Equilibrium Constant Kp.
From www.coursehero.com
[Solved] Experiments have shown that the equilibrium constant Kp for What Is Equilibrium Constant Kp equilibrium constant kp definition. When a reaction is at equilibrium, the forward and reverse reaction rate are. Aa + bb ⇆ cc + dd. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that.. What Is Equilibrium Constant Kp.
From www.researchgate.net
Equilibrium constant Kp as a function of temperature for some reactions What Is Equilibrium Constant Kp the equilibrium constant, k, is a number that describes the relationship between the number of products and. It covers an explanation of the. equilibrium constant kp definition. Let’s see an example of calculating. k p and k c are the equilibrium constant of an ideal gaseous mixture. this page explains equilibrium constants expressed in terms of. What Is Equilibrium Constant Kp.
From www.youtube.com
Calculating Equilibrium Constant, Kp YouTube What Is Equilibrium Constant Kp for a general reaction of gases, the equilibrium constant kp can be represented as: an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. k p and k c are the equilibrium constant of an ideal gaseous mixture. equilibrium constant kp definition. K p. What Is Equilibrium Constant Kp.
From byjus.com
The equilibrium constant kp for the reaction H2g) + CO2,g) gives H2O(g What Is Equilibrium Constant Kp K p is equilibrium constant used when equilibrium. When a reaction is at equilibrium, the forward and reverse reaction rate are. the equilibrium constant, k, is a number that describes the relationship between the number of products and. k p and k c are the equilibrium constant of an ideal gaseous mixture. Let’s see an example of calculating.. What Is Equilibrium Constant Kp.
From www.alamy.com
The equilibrium constant Kp expression of the reaction Stock Vector What Is Equilibrium Constant Kp the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. K p is equilibrium constant used when equilibrium. Let’s see an example of calculating. the equilibrium constant, k, is a number that describes the relationship between the number of products and. When a reaction is at equilibrium, the forward and. What Is Equilibrium Constant Kp.
From thechemistrynotes.com
Equilibrium Constant Definition, Characteristics, and Calculations What Is Equilibrium Constant Kp equilibrium constant kp definition. When a reaction is at equilibrium, the forward and reverse reaction rate are. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. Aa + bb ⇆ cc + dd. Let’s see an example of calculating. k p and k c. What Is Equilibrium Constant Kp.
From www.tes.com
Equilibrium Constant Expression (Kc Kp and units) ALevel Chemistry What Is Equilibrium Constant Kp the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. for a general reaction of gases, the equilibrium constant kp can be represented as: Aa + bb ⇆ cc + dd. When a reaction is at equilibrium, the forward and reverse reaction rate are. this page explains equilibrium constants. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT Chapter 15 Chemical Equilibrium PowerPoint Presentation, free What Is Equilibrium Constant Kp When a reaction is at equilibrium, the forward and reverse reaction rate are. equilibrium constant kp definition. Let’s see an example of calculating. It covers an explanation of the. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. this page explains equilibrium constants expressed in terms of partial. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID What Is Equilibrium Constant Kp It covers an explanation of the. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. equilibrium constant kp definition. K p is equilibrium constant used when equilibrium. k. What Is Equilibrium Constant Kp.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership What Is Equilibrium Constant Kp an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. Aa + bb ⇆ cc + dd. Let’s see an example of calculating. K p is equilibrium constant used when equilibrium. k p and k c are the equilibrium constant of an ideal gaseous mixture. . What Is Equilibrium Constant Kp.
From www.youtube.com
Understanding The Equilibrium Constant, Kp (A2 Chemistry) YouTube What Is Equilibrium Constant Kp an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. Let’s see an example of calculating. equilibrium constant kp definition. Aa + bb ⇆ cc + dd. for a general reaction of gases, the equilibrium constant kp can be represented as: It covers an explanation. What Is Equilibrium Constant Kp.
From www.youtube.com
Equilibrium Constant for Gaseous Reactions Kp YouTube What Is Equilibrium Constant Kp Let’s see an example of calculating. When a reaction is at equilibrium, the forward and reverse reaction rate are. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. k p and k c. What Is Equilibrium Constant Kp.
From materialoster.z21.web.core.windows.net
Equilibrium Constant K Chemistry Definition What Is Equilibrium Constant Kp Aa + bb ⇆ cc + dd. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. When a reaction is at equilibrium, the forward and reverse reaction rate are. equilibrium constant kp definition. k p and k c are the equilibrium constant of an. What Is Equilibrium Constant Kp.
From www.vrogue.co
The Equilibrium Constant Kp Of The Reaction 2so2 O2 2 vrogue.co What Is Equilibrium Constant Kp K p is equilibrium constant used when equilibrium. equilibrium constant kp definition. the equilibrium constant, k, is a number that describes the relationship between the number of products and. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. When a reaction is at equilibrium, the forward and reverse. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download What Is Equilibrium Constant Kp Let’s see an example of calculating. equilibrium constant kp definition. It covers an explanation of the. When a reaction is at equilibrium, the forward and reverse reaction rate are. k p and k c are the equilibrium constant of an ideal gaseous mixture. the equilibrium constant of pressure gives the ratio of pressure of products over reactants. What Is Equilibrium Constant Kp.
From www.youtube.com
The equilibrium constant Kp YouTube What Is Equilibrium Constant Kp this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. for a general reaction of gases, the equilibrium constant kp can be represented as: It covers an explanation of the. k p and k c are the equilibrium constant of an ideal gaseous mixture. Let’s see an example of calculating. When a. What Is Equilibrium Constant Kp.
From www.vrogue.co
The Equilibrium Constant Kp For The Reaction 2so2 O2 vrogue.co What Is Equilibrium Constant Kp an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. the equilibrium constant, k, is a number that describes the relationship between the number of products and. the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that.. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT Thermodynamic equilibrium constant K PowerPoint Presentation What Is Equilibrium Constant Kp the equilibrium constant, k, is a number that describes the relationship between the number of products and. K p is equilibrium constant used when equilibrium. equilibrium constant kp definition. It covers an explanation of the. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Let’s see an example of calculating. . What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download What Is Equilibrium Constant Kp the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. equilibrium constant kp definition. When a reaction is at equilibrium, the forward and reverse reaction rate are. k p and k c are the equilibrium constant of an ideal gaseous mixture. Aa + bb ⇆ cc + dd. K. What Is Equilibrium Constant Kp.
From www.slideserve.com
PPT Homogeneous Gaseous Equilibria PowerPoint Presentation, free What Is Equilibrium Constant Kp for a general reaction of gases, the equilibrium constant kp can be represented as: It covers an explanation of the. Aa + bb ⇆ cc + dd. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal. What Is Equilibrium Constant Kp.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps What Is Equilibrium Constant Kp the equilibrium constant of pressure gives the ratio of pressure of products over reactants for a reaction that. an equilibrium constant calculated from partial pressures (\(k_p\)) is related to \(k\) by the ideal gas constant (\(r\)), the temperature (\(t\)), and. equilibrium constant kp definition. for a general reaction of gases, the equilibrium constant kp can be. What Is Equilibrium Constant Kp.
From www.pinterest.com
Learn how to calculate an equilibrium constant Kp. Chemistry Help, High What Is Equilibrium Constant Kp When a reaction is at equilibrium, the forward and reverse reaction rate are. It covers an explanation of the. Let’s see an example of calculating. Aa + bb ⇆ cc + dd. for a general reaction of gases, the equilibrium constant kp can be represented as: equilibrium constant kp definition. k p and k c are the. What Is Equilibrium Constant Kp.
From slideplayer.com
Chapter 15 Chemical Equilibrium John A. Schreifels Chemistry ppt download What Is Equilibrium Constant Kp for a general reaction of gases, the equilibrium constant kp can be represented as: Aa + bb ⇆ cc + dd. It covers an explanation of the. equilibrium constant kp definition. this page explains equilibrium constants expressed in terms of partial pressures of gases, k p. K p is equilibrium constant used when equilibrium. When a reaction. What Is Equilibrium Constant Kp.