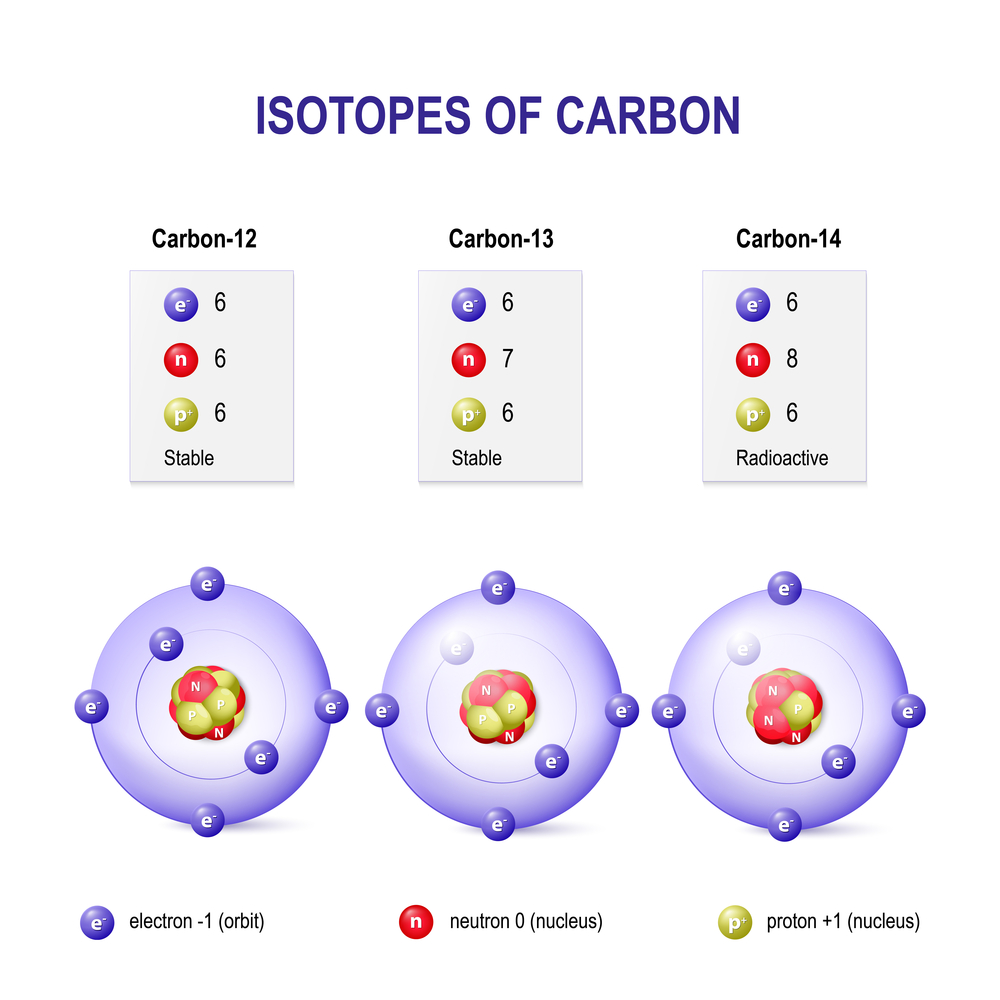
Carbon Vs Carbon 12 . The main difference between them lies in their atomic mass. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Carbon occurs naturally in three isotopes: He says that a practical solution is to choose a number that is. Every element has its own number of isotopes. The addition of even one neutron can dramatically change an isotope’s properties.
from www.scienceabc.com
Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The addition of even one neutron can dramatically change an isotope’s properties. He says that a practical solution is to choose a number that is. Carbon occurs naturally in three isotopes: The main difference between them lies in their atomic mass. Every element has its own number of isotopes.
Isotopes Definition, Explanation, Properties And Examples
Carbon Vs Carbon 12 Every element has its own number of isotopes. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. Every element has its own number of isotopes. The main difference between them lies in their atomic mass. Carbon occurs naturally in three isotopes: The addition of even one neutron can dramatically change an isotope’s properties.
From www.scienceabc.com
Isotopes Definition, Explanation, Properties And Examples Carbon Vs Carbon 12 He says that a practical solution is to choose a number that is. The main difference between them lies in their atomic mass. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes. The addition of even one. Carbon Vs Carbon 12.
From sitn.hms.harvard.edu
Unexpected Lessons Learned from MidCentury Atomic Bomb Explosions Carbon Vs Carbon 12 The main difference between them lies in their atomic mass. The addition of even one neutron can dramatically change an isotope’s properties. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. Carbon. Carbon Vs Carbon 12.
From www.youtube.com
NOMENCLATURE OF CARBON COMPOUNDS YouTube Carbon Vs Carbon 12 The main difference between them lies in their atomic mass. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes. Carbon occurs naturally in three isotopes: The addition of even one neutron can dramatically change an isotope’s properties.. Carbon Vs Carbon 12.
From treasury-management.com
Carbonomics 101 Carbon neutral vs. net zero why the difference Carbon Vs Carbon 12 The addition of even one neutron can dramatically change an isotope’s properties. Carbon occurs naturally in three isotopes: The main difference between them lies in their atomic mass. Every element has its own number of isotopes. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons.. Carbon Vs Carbon 12.
From www.pinterest.co.kr
Carbon is one of the most abundant elements and forms a very large Carbon Vs Carbon 12 He says that a practical solution is to choose a number that is. The main difference between them lies in their atomic mass. Carbon occurs naturally in three isotopes: Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The addition of even one neutron can. Carbon Vs Carbon 12.
From www.youtube.com
Carbon12 Meaning YouTube Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. The main difference between them lies in their atomic mass. Every element has its own number of isotopes. Carbon occurs naturally in three. Carbon Vs Carbon 12.
From www.dreamstime.com
Carbon, Atom Model of Carbon12 with 6 Protons, 6 Neutrons and 6 Carbon Vs Carbon 12 The addition of even one neutron can dramatically change an isotope’s properties. Every element has its own number of isotopes. He says that a practical solution is to choose a number that is. Carbon occurs naturally in three isotopes: Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which. Carbon Vs Carbon 12.
From www.differencebetween.com
Difference Between Carbon 12 and Carbon 14 Compare the Difference Carbon Vs Carbon 12 The addition of even one neutron can dramatically change an isotope’s properties. Every element has its own number of isotopes. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. Carbon occurs naturally. Carbon Vs Carbon 12.
From www.vecteezy.com
Carbon symbol. Chemical element of the periodic table. Vector Carbon Vs Carbon 12 The addition of even one neutron can dramatically change an isotope’s properties. Every element has its own number of isotopes. Carbon occurs naturally in three isotopes: He says that a practical solution is to choose a number that is. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which. Carbon Vs Carbon 12.
From tatianafersstuart.blogspot.com
Why Carbon 12 is Taken as Standard Carbon Vs Carbon 12 Carbon occurs naturally in three isotopes: Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The addition of even one neutron can dramatically change an isotope’s properties. Every element has its own number of isotopes. The main difference between them lies in their atomic mass.. Carbon Vs Carbon 12.
From askanydifference.com
Carbon 12 vs Carbon 14 Difference and Comparison Carbon Vs Carbon 12 Every element has its own number of isotopes. Carbon occurs naturally in three isotopes: Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The main difference between them lies in their atomic mass. The addition of even one neutron can dramatically change an isotope’s properties.. Carbon Vs Carbon 12.
From www.youtube.com
Carbon found nature as a mixture of C12 and C13. The average atomic Carbon Vs Carbon 12 Carbon occurs naturally in three isotopes: The main difference between them lies in their atomic mass. Every element has its own number of isotopes. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that. Carbon Vs Carbon 12.
From stock.adobe.com
Isotopes of Carbon. Three natural isotopes of carbon. Atomic Structure Carbon Vs Carbon 12 He says that a practical solution is to choose a number that is. The main difference between them lies in their atomic mass. Carbon occurs naturally in three isotopes: Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of. Carbon Vs Carbon 12.
From thecontentauthority.com
Carbone vs Carbon Do These Mean The Same? How To Use Them Carbon Vs Carbon 12 The addition of even one neutron can dramatically change an isotope’s properties. Every element has its own number of isotopes. The main difference between them lies in their atomic mass. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution. Carbon Vs Carbon 12.
From religionnews.com
Carbon12 announces presale and public launch timelines of C12 token Carbon Vs Carbon 12 The main difference between them lies in their atomic mass. Every element has its own number of isotopes. Carbon occurs naturally in three isotopes: The addition of even one neutron can dramatically change an isotope’s properties. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons.. Carbon Vs Carbon 12.
From www.alamy.com
Carbon isotopes. Atomic Structure from Carbon12 to Carbon14. Atomic Carbon Vs Carbon 12 The main difference between them lies in their atomic mass. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. Every element has its own number of isotopes. Carbon occurs naturally in three. Carbon Vs Carbon 12.
From terapiayrehabilitacionfisica.com
The Atomic Labyrinth Deciphering the Carbon12 Nucleus Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. Every element has its own number of isotopes. The addition of even one neutron can dramatically change an isotope’s properties. The main difference. Carbon Vs Carbon 12.
From www.youtube.com
Primary carbon secondary carbon tertiary carbon Types of Carbons Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Carbon occurs naturally in three isotopes: The addition of even one neutron can dramatically change an isotope’s properties. He says that a practical solution is to choose a number that is. The main difference between them. Carbon Vs Carbon 12.
From blog.thepipingmart.com
12L14 vs Low Carbon Steel What's the Difference Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes. The main difference between them lies in their atomic mass. Carbon occurs naturally in three isotopes: He says that a practical solution is to choose a number that. Carbon Vs Carbon 12.
From www.vecteezy.com
Carbon symbol. Chemical element of the periodic table. Vector Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. Carbon occurs naturally in three isotopes: The addition of even one neutron can dramatically change an isotope’s properties. The main difference between them. Carbon Vs Carbon 12.
From ciaraancegreer.blogspot.com
Mass of One Atom of Carbon 12 CiaraanceGreer Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes. The addition of even one neutron can dramatically change an isotope’s properties. Carbon occurs naturally in three isotopes: The main difference between them lies in their atomic mass.. Carbon Vs Carbon 12.
From www.slideserve.com
PPT Carbon Dating PowerPoint Presentation, free download ID5849821 Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution is to choose a number that is. The addition of even one neutron can dramatically change an isotope’s properties. The main difference between them lies in their atomic mass. Every. Carbon Vs Carbon 12.
From differencebetweenz.com
Difference between Carbon12 and Carbon14 Difference Betweenz Carbon Vs Carbon 12 Every element has its own number of isotopes. The main difference between them lies in their atomic mass. Carbon occurs naturally in three isotopes: The addition of even one neutron can dramatically change an isotope’s properties. He says that a practical solution is to choose a number that is. Carbon 12, which has 6 neutrons (plus 6 protons equals 12),. Carbon Vs Carbon 12.
From www.pinterest.com
Carbon12 Electron configuration, Carbon element, Atom Carbon Vs Carbon 12 Carbon occurs naturally in three isotopes: Every element has its own number of isotopes. The addition of even one neutron can dramatically change an isotope’s properties. He says that a practical solution is to choose a number that is. The main difference between them lies in their atomic mass. Carbon 12, which has 6 neutrons (plus 6 protons equals 12),. Carbon Vs Carbon 12.
From ge.investments
What are the differences among carbon neutral, net zero, and carbon Carbon Vs Carbon 12 The addition of even one neutron can dramatically change an isotope’s properties. Carbon occurs naturally in three isotopes: Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The main difference between them lies in their atomic mass. He says that a practical solution is to. Carbon Vs Carbon 12.
From www.alamy.com
Carbon12. Diagram showing the nuclear composition and electron Carbon Vs Carbon 12 He says that a practical solution is to choose a number that is. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The addition of even one neutron can dramatically change an isotope’s properties. Carbon occurs naturally in three isotopes: Every element has its own. Carbon Vs Carbon 12.
From www.differencebetween.com
Difference Between Carbon 12 and Carbon 14 Compare the Difference Carbon Vs Carbon 12 Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The addition of even one neutron can dramatically change an isotope’s properties. Carbon occurs naturally in three isotopes: The main difference between them lies in their atomic mass. He says that a practical solution is to. Carbon Vs Carbon 12.
From aamorinox.com
Stainless Steel vs Carbon Steel Aamor Inox Carbon Vs Carbon 12 The addition of even one neutron can dramatically change an isotope’s properties. Carbon occurs naturally in three isotopes: He says that a practical solution is to choose a number that is. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The main difference between them. Carbon Vs Carbon 12.
From www.smorescience.com
How Accurate is Carbon Dating? Smore Science Magazine Carbon Vs Carbon 12 He says that a practical solution is to choose a number that is. The addition of even one neutron can dramatically change an isotope’s properties. Every element has its own number of isotopes. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. The main difference. Carbon Vs Carbon 12.
From chembites.org
Tracing carbon atoms through the galaxy Chembites Carbon Vs Carbon 12 Carbon occurs naturally in three isotopes: Every element has its own number of isotopes. The addition of even one neutron can dramatically change an isotope’s properties. The main difference between them lies in their atomic mass. He says that a practical solution is to choose a number that is. Carbon 12, which has 6 neutrons (plus 6 protons equals 12),. Carbon Vs Carbon 12.
From www.goodscience.com.au
Radioactivity Good Science Carbon Vs Carbon 12 Every element has its own number of isotopes. Carbon occurs naturally in three isotopes: He says that a practical solution is to choose a number that is. The addition of even one neutron can dramatically change an isotope’s properties. The main difference between them lies in their atomic mass. Carbon 12, which has 6 neutrons (plus 6 protons equals 12),. Carbon Vs Carbon 12.
From www.abc.net.au
A date with carbon › Bernie's Basics (ABC Science) Carbon Vs Carbon 12 Carbon occurs naturally in three isotopes: The addition of even one neutron can dramatically change an isotope’s properties. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. Every element has its own number of isotopes. The main difference between them lies in their atomic mass.. Carbon Vs Carbon 12.
From www.youtube.com
What is the difference between carbon12 and 13? YouTube Carbon Vs Carbon 12 Every element has its own number of isotopes. The main difference between them lies in their atomic mass. The addition of even one neutron can dramatically change an isotope’s properties. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons. He says that a practical solution. Carbon Vs Carbon 12.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Carbon12 Carbon Vs Carbon 12 Every element has its own number of isotopes. Carbon occurs naturally in three isotopes: The main difference between them lies in their atomic mass. He says that a practical solution is to choose a number that is. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8. Carbon Vs Carbon 12.
From pediaa.com
Difference Between Carbon 12 and Carbon 14 Definition, Structure Carbon Vs Carbon 12 The main difference between them lies in their atomic mass. Carbon occurs naturally in three isotopes: Every element has its own number of isotopes. The addition of even one neutron can dramatically change an isotope’s properties. Carbon 12, which has 6 neutrons (plus 6 protons equals 12), carbon 13, which has 7 neutrons, and carbon 14, which has 8 neutrons.. Carbon Vs Carbon 12.