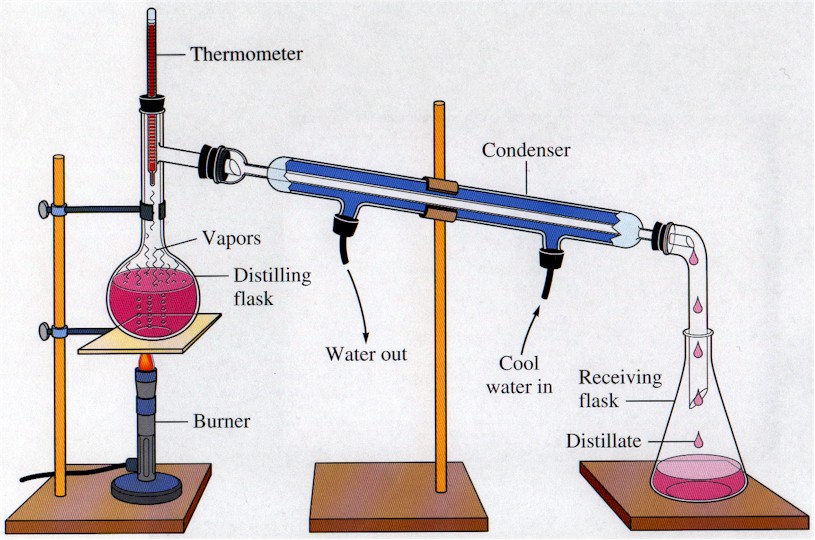
Vacuum Distillation Apparatus . Vacuum distillation is an important process in the chemical and pharmaceutical industry. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. a completed distillation apparatus is shown in figure 5.25. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. No parts should be able to jiggle or they are not adequately clamped. how does vacuum distillation work? A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. what is vacuum distillation? Boiling commences when the vapor pressure of a liquid or solution equals the external.
from
a completed distillation apparatus is shown in figure 5.25. No parts should be able to jiggle or they are not adequately clamped. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Vacuum distillation is an important process in the chemical and pharmaceutical industry. what is vacuum distillation? Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. how does vacuum distillation work? Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the vapor pressure of a liquid or solution equals the external.
Vacuum Distillation Apparatus what is vacuum distillation? No parts should be able to jiggle or they are not adequately clamped. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. how does vacuum distillation work? Boiling commences when the vapor pressure of a liquid or solution equals the external. what is vacuum distillation? a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Vacuum distillation is an important process in the chemical and pharmaceutical industry. a completed distillation apparatus is shown in figure 5.25. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable.
From
Vacuum Distillation Apparatus a completed distillation apparatus is shown in figure 5.25. Vacuum distillation is an important process in the chemical and pharmaceutical industry. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. how does vacuum distillation work?. Vacuum Distillation Apparatus.
From lnhksysb.en.made-in-china.com
Vacuum Distillation Apparatus (ASTM D1160) China Vacuum Distillation Apparatus and Reduced Vacuum Distillation Apparatus Vacuum distillation is an important process in the chemical and pharmaceutical industry. what is vacuum distillation? a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. how does vacuum distillation work? Although it may appear to be. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus what is vacuum distillation? a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Boiling commences when the vapor pressure of a liquid or solution equals the external. Although it may appear to be a closed system (which. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Boiling commences when the vapor pressure of a liquid or solution equals the external. Vacuum distillation is an important process in the chemical and pharmaceutical industry. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. No parts. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Vacuum distillation is an important process in the chemical and pharmaceutical industry. a completed distillation apparatus is shown in figure 5.25. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. Vacuum distillation is a technique for the purification. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Vacuum distillation is an important process in the chemical and pharmaceutical industry. No parts should be able to jiggle or they are not adequately clamped. a completed distillation. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. No parts should be able to jiggle or they are not adequately clamped. how does vacuum distillation work? a vacuum distillation is used when the boiling. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus how does vacuum distillation work? a completed distillation apparatus is shown in figure 5.25. what is vacuum distillation? Boiling commences when the vapor pressure of a liquid or solution equals the external. Vacuum distillation is an important process in the chemical and pharmaceutical industry. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation. Vacuum Distillation Apparatus.
From parkesscientific.com
Gecil Minidist 1160 Version 7, Fully Automatic Vacuum Distillation Apparatus Parkes Scientific Vacuum Distillation Apparatus Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is used when the boiling point of the compound (or the solvent). Vacuum Distillation Apparatus.
From chinainstrument.en.made-in-china.com
Automatic ASTM D1160 Vacuum Distillation Apparatus China Vacuum Distillation ASTM D1160 and Vacuum Distillation Apparatus what is vacuum distillation? Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. No parts should be able to jiggle or they are. Vacuum Distillation Apparatus.
From www.dennisdeal.com
1000mL 24/40 Distillation Apparatus Vacuum Distill Kit Vigreux Column Vacuum Distillation Apparatus how does vacuum distillation work? A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a completed distillation apparatus is shown in figure 5.25. No parts should be able to jiggle or they are not adequately clamped. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus how does vacuum distillation work? what is vacuum distillation? Vacuum distillation is an important process in the chemical and pharmaceutical industry. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. a completed distillation apparatus is shown in figure 5.25. No parts should be able to jiggle or they are not adequately clamped.. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. what is vacuum distillation? A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of. Vacuum Distillation Apparatus.
From www.researchgate.net
2 Vacuum distillation Apparatus. Download Scientific Diagram Vacuum Distillation Apparatus No parts should be able to jiggle or they are not adequately clamped. Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. a. Vacuum Distillation Apparatus.
From www.sz-pharma.com
Customized SS 304 Vacuum Distillation Equipment EvaporatorPHARMA Vacuum Distillation Apparatus what is vacuum distillation? Boiling commences when the vapor pressure of a liquid or solution equals the external. how does vacuum distillation work? Vacuum distillation is an important process in the chemical and pharmaceutical industry. No parts should be able to jiggle or they are not adequately clamped. A vacuum distillation apparatus is shown in figure 5.50, using. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Boiling commences when the vapor pressure of a liquid or solution equals the external. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. how does vacuum distillation work? No parts should be able to jiggle or they are not adequately clamped. Although it may appear to be a closed system (which would be dangerous. Vacuum Distillation Apparatus.
From mavink.com
Vacuum Distillation Set Up Vacuum Distillation Apparatus No parts should be able to jiggle or they are not adequately clamped. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. a completed distillation apparatus is shown in figure 5.25. a vacuum distillation is used when. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. No parts should be able to jiggle or they are not adequately clamped. Although it may appear to be a closed system (which would be dangerous to heat),. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. what is vacuum distillation? how does vacuum distillation work? Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus No parts should be able to jiggle or they are not adequately clamped. Boiling commences when the vapor pressure of a liquid or solution equals the external. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. a vacuum. Vacuum Distillation Apparatus.
From tillescenter.org
Precision 14/20 Vacuum Jacketed Short Path Distillation Apparatus tillescenter Glassware Vacuum Distillation Apparatus Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. Boiling commences when the vapor pressure of a liquid or solution equals the external. how does vacuum distillation work? a vacuum distillation is used when the. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus a completed distillation apparatus is shown in figure 5.25. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Boiling commences when the. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Boiling commences when the vapor pressure of a liquid or solution equals the external. a completed distillation apparatus is shown in figure 5.25. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. what is vacuum distillation?. Vacuum Distillation Apparatus.
From www.lab-testkit.com
GD0165A Vacuum Distillation Apparatus BIntroduction This instrument is designed and made as Vacuum Distillation Apparatus Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. No parts should be able to jiggle or they are not adequately clamped. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. how does. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus No parts should be able to jiggle or they are not adequately clamped. how does vacuum distillation work? a completed distillation apparatus is shown in figure 5.25. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. A. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus how does vacuum distillation work? a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere. Vacuum Distillation Apparatus.
From chem.libretexts.org
5.4C StepbyStep Procedures for Vacuum Distillation Chemistry LibreTexts Vacuum Distillation Apparatus Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. what is vacuum distillation? Boiling commences when the vapor pressure of a liquid or solution equals the external. No parts should be able to jiggle or they. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus No parts should be able to jiggle or they are not adequately clamped. a completed distillation apparatus is shown in figure 5.25. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. Boiling commences when the vapor. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Boiling commences when the vapor pressure of a liquid or solution equals the external. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Boiling commences when the vapor pressure of a liquid or solution equals the external. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. a completed distillation apparatus is shown in figure 5.25. Vacuum distillation is an important. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus how does vacuum distillation work? a completed distillation apparatus is shown in figure 5.25. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. No parts should be able to jiggle or they are not adequately clamped. . Vacuum Distillation Apparatus.
From labnique.com
Glass Vacuum Filtration Distillation Apparatus with Compact Vacuum Pum labnique Vacuum Distillation Apparatus Vacuum distillation is an important process in the chemical and pharmaceutical industry. A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. Vacuum distillation is a technique for the purification or separation of liquids or solvents with a boiling point above 150 ºc, or with a lower boiling point but which are thermally unstable. a. Vacuum Distillation Apparatus.
From www.sz-pharma.com
Customized SS 304 Vacuum Distillation Equipment EvaporatorPHARMA Vacuum Distillation Apparatus A vacuum distillation apparatus is shown in figure 5.50, using a simple distillation setup. No parts should be able to jiggle or they are not adequately clamped. how does vacuum distillation work? a completed distillation apparatus is shown in figure 5.25. Vacuum distillation is an important process in the chemical and pharmaceutical industry. Vacuum distillation is a technique. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus Boiling commences when the vapor pressure of a liquid or solution equals the external. a completed distillation apparatus is shown in figure 5.25. Although it may appear to be a closed system (which would be dangerous to heat), the system is actually open to the atmosphere at the arm in the vacuum adapter. No parts should be able to. Vacuum Distillation Apparatus.
From
Vacuum Distillation Apparatus what is vacuum distillation? No parts should be able to jiggle or they are not adequately clamped. a vacuum distillation is used when the boiling point of the compound (or the solvent) is too high (t>150 o c) in order to distill the compound (or the solvent. Boiling commences when the vapor pressure of a liquid or solution. Vacuum Distillation Apparatus.