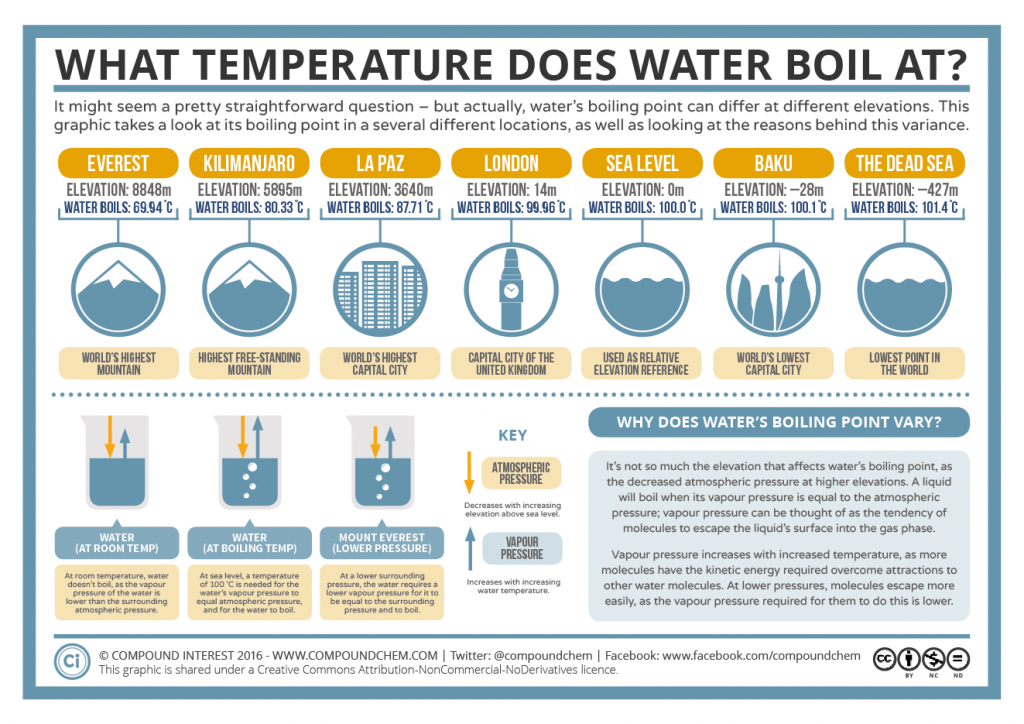
What Happens To Boiling Point Of Liquid . Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. When the vapour pressure equals atmospheric pressure, the liquid boils. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The boiling point is the temperature at which boiling occurs for a specific liquid. The lower the pressure of a gas above a liquid, the lower the temperature at. Normally when we boil a liquid, we do so at atmospheric pressure. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. At a pressure greater than 1 atm,. For example, for water, the boiling point is 100ºc at. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The impurities lower the vapor pressure of the liquid. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it.
from www.compoundchem.com
Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The lower the pressure of a gas above a liquid, the lower the temperature at. As elevation increases, atmospheric pressure decreases because air is less dense at higher. At a pressure greater than 1 atm,. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. For example, for water, the boiling point is 100ºc at. Normally when we boil a liquid, we do so at atmospheric pressure. When the vapour pressure equals atmospheric pressure, the liquid boils. The boiling point is the temperature at which boiling occurs for a specific liquid. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure.
What Temperature Does Water Boil At? Boiling Point & Elevation
What Happens To Boiling Point Of Liquid For example, for water, the boiling point is 100ºc at. Normally when we boil a liquid, we do so at atmospheric pressure. At a pressure greater than 1 atm,. The lower the pressure of a gas above a liquid, the lower the temperature at. When the vapour pressure equals atmospheric pressure, the liquid boils. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. The boiling point is the temperature at which boiling occurs for a specific liquid. For example, for water, the boiling point is 100ºc at. The impurities lower the vapor pressure of the liquid. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure.
From scienceisntscary.wordpress.com
Boiling point Ease Into Science What Happens To Boiling Point Of Liquid The boiling point is the temperature at which boiling occurs for a specific liquid. The lower the pressure of a gas above a liquid, the lower the temperature at. When the vapour pressure equals atmospheric pressure, the liquid boils. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Contaminants or other nonvolatile molecules in a liquid. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Happens To Boiling Point Of Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. As elevation increases, atmospheric pressure decreases because air is less dense at higher. At a pressure greater than 1 atm,. Normally when we boil a liquid, we do so at atmospheric pressure. The lower. What Happens To Boiling Point Of Liquid.
From www.youtube.com
Method To Determine Boiling Point Of A Liquid Basic Principles and What Happens To Boiling Point Of Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The boiling point is the temperature at which boiling occurs for a specific liquid. Contaminants or other nonvolatile molecules in. What Happens To Boiling Point Of Liquid.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Happens To Boiling Point Of Liquid The lower the pressure of a gas above a liquid, the lower the temperature at. When the vapour pressure equals atmospheric pressure, the liquid boils. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. A liquid boils at a temperature at which its vapor pressure is equal to the pressure. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT boiling point PowerPoint Presentation, free download ID2402961 What Happens To Boiling Point Of Liquid Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. At a pressure greater than 1 atm,. When the. What Happens To Boiling Point Of Liquid.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps What Happens To Boiling Point Of Liquid For example, for water, the boiling point is 100ºc at. Normally when we boil a liquid, we do so at atmospheric pressure. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. A liquid boils at a temperature. What Happens To Boiling Point Of Liquid.
From www.youtube.com
Boiling Points of Liquids, Chemistry Lecture Sabaq.pk YouTube What Happens To Boiling Point Of Liquid Normally when we boil a liquid, we do so at atmospheric pressure. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. The boiling point is the temperature at which boiling occurs for a specific liquid. As elevation increases, atmospheric pressure decreases because air is less dense at higher. When the. What Happens To Boiling Point Of Liquid.
From www.youtube.com
The boiling point of liquid hydrogen is 203 K at atmospheric pressure What Happens To Boiling Point Of Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. When the vapour pressure equals atmospheric pressure, the liquid boils. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The boiling point. What Happens To Boiling Point Of Liquid.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Happens To Boiling Point Of Liquid For example, for water, the boiling point is 100ºc at. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. The lower the. What Happens To Boiling Point Of Liquid.
From www.youtube.com
Chemistry Animation Salt in Boiling Water.wmv YouTube What Happens To Boiling Point Of Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. As elevation increases, atmospheric pressure decreases because air is less dense at higher.. What Happens To Boiling Point Of Liquid.
From diagramfricanofc.z21.web.core.windows.net
Initial Boiling Point And Final Boiling Point What Happens To Boiling Point Of Liquid Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The impurities lower the vapor pressure of the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. At a pressure greater than 1. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT States of Matter Phase Change PowerPoint Presentation ID1115834 What Happens To Boiling Point Of Liquid As elevation increases, atmospheric pressure decreases because air is less dense at higher. The lower the pressure of a gas above a liquid, the lower the temperature at. The boiling point is the temperature at which boiling occurs for a specific liquid. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the. What Happens To Boiling Point Of Liquid.
From www.youtube.com
What is boiling point what is the boiling point Factors affecting What Happens To Boiling Point Of Liquid At a pressure greater than 1 atm,. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Boiling point, temperature at which the pressure exerted by the surroundings upon a. What Happens To Boiling Point Of Liquid.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Happens To Boiling Point Of Liquid The lower the pressure of a gas above a liquid, the lower the temperature at. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation.. What Happens To Boiling Point Of Liquid.
From spikaph.blogspot.com
Boiling Point Of Water Boiling point of water YouTube For What Happens To Boiling Point Of Liquid At a pressure greater than 1 atm,. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. The lower the pressure of a gas above a liquid, the lower the temperature at. The impurities lower the vapor pressure of the liquid. When the vapour pressure equals atmospheric pressure, the. What Happens To Boiling Point Of Liquid.
From general.chemistrysteps.com
Boiling Point Elevation Chemistry Steps What Happens To Boiling Point Of Liquid At a pressure greater than 1 atm,. When the vapour pressure equals atmospheric pressure, the liquid boils. The impurities lower the vapor pressure of the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Although. What Happens To Boiling Point Of Liquid.
From www.physicsfox.org
Melting & Boiling • Matter • Physics Fox What Happens To Boiling Point Of Liquid When the vapour pressure equals atmospheric pressure, the liquid boils. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Normally when we boil a liquid, we do so at. What Happens To Boiling Point Of Liquid.
From www.istockphoto.com
Boiling And Evaporation Freezing And Melting Points Of Water Stock What Happens To Boiling Point Of Liquid At a pressure greater than 1 atm,. As elevation increases, atmospheric pressure decreases because air is less dense at higher. When the vapour pressure equals atmospheric pressure, the liquid boils. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Although we usually cite the normal boiling point of a liquid,. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT Chapter 13 continued… PowerPoint Presentation, free download ID What Happens To Boiling Point Of Liquid As elevation increases, atmospheric pressure decreases because air is less dense at higher. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. For example, for water, the boiling point is 100ºc at. If this pressure is the standard pressure of 1 atm (101.3. What Happens To Boiling Point Of Liquid.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Happens To Boiling Point Of Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature. What Happens To Boiling Point Of Liquid.
From www.vedantu.com
Boiling Point Elevation Learn Important Terms and Concepts What Happens To Boiling Point Of Liquid Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. At a pressure greater than 1 atm,. The lower the pressure of a gas above a liquid, the lower the temperature at. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas. What Happens To Boiling Point Of Liquid.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Happens To Boiling Point Of Liquid As elevation increases, atmospheric pressure decreases because air is less dense at higher. The boiling point is the temperature at which boiling occurs for a specific liquid. For example, for water, the boiling point is 100ºc at. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. At a pressure greater. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT Ch. 13 States of Matter PowerPoint Presentation, free download What Happens To Boiling Point Of Liquid For example, for water, the boiling point is 100ºc at. When the vapour pressure equals atmospheric pressure, the liquid boils. The boiling point is the temperature at which boiling occurs for a specific liquid. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Although we usually cite the normal boiling. What Happens To Boiling Point Of Liquid.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Happens To Boiling Point Of Liquid Normally when we boil a liquid, we do so at atmospheric pressure. The impurities lower the vapor pressure of the liquid. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in. What Happens To Boiling Point Of Liquid.
From sciencenotes.org
What Are the Bubbles in Boiling Water? What Happens To Boiling Point Of Liquid When the vapour pressure equals atmospheric pressure, the liquid boils. Normally when we boil a liquid, we do so at atmospheric pressure. The lower the pressure of a gas above a liquid, the lower the temperature at. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The impurities lower the. What Happens To Boiling Point Of Liquid.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Happens To Boiling Point Of Liquid Normally when we boil a liquid, we do so at atmospheric pressure. At a pressure greater than 1 atm,. The impurities lower the vapor pressure of the liquid. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. The boiling point is the temperature at which boiling occurs for a specific. What Happens To Boiling Point Of Liquid.
From studylib.net
Boiling Point = Temperature at which a liquid turns into a gas What Happens To Boiling Point Of Liquid The boiling point is the temperature at which boiling occurs for a specific liquid. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. For example, for water, the boiling point is. What Happens To Boiling Point Of Liquid.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Happens To Boiling Point Of Liquid As elevation increases, atmospheric pressure decreases because air is less dense at higher. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;.. What Happens To Boiling Point Of Liquid.
From lyndajguilleno.blob.core.windows.net
What Happens To Water When It Reaches Its Boiling Point at What Happens To Boiling Point Of Liquid A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Although we usually cite the normal boiling point of a liquid, the actual boiling point depends on the pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Boiling. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 What Happens To Boiling Point Of Liquid The lower the pressure of a gas above a liquid, the lower the temperature at. Normally when we boil a liquid, we do so at atmospheric pressure. The impurities lower the vapor pressure of the liquid. The boiling point is the temperature at which boiling occurs for a specific liquid. Contaminants or other nonvolatile molecules in a liquid increase its. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT Chapter 10 States of Matter PowerPoint Presentation, free What Happens To Boiling Point Of Liquid Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure exerted by the vapor of the liquid;. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Normally when. What Happens To Boiling Point Of Liquid.
From jsmithmoore.com
Boiling point of ethanol celsius What Happens To Boiling Point Of Liquid When the vapour pressure equals atmospheric pressure, the liquid boils. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The boiling point is. What Happens To Boiling Point Of Liquid.
From www.youtube.com
Binary Boiling Point Diagram of a LiquidLiquid Mixture YouTube What Happens To Boiling Point Of Liquid The lower the pressure of a gas above a liquid, the lower the temperature at. At a pressure greater than 1 atm,. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation. Boiling point, temperature at which the pressure exerted by the surroundings upon a liquid is equaled by the pressure. What Happens To Boiling Point Of Liquid.
From www.slideserve.com
PPT The Nature of Matter PowerPoint Presentation, free download ID What Happens To Boiling Point Of Liquid As elevation increases, atmospheric pressure decreases because air is less dense at higher. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The lower the pressure of a gas above a liquid, the lower the temperature at. When the vapour pressure equals atmospheric pressure, the liquid boils. Normally when we. What Happens To Boiling Point Of Liquid.
From www.animalia-life.club
Boiling Point Of Water For Kids What Happens To Boiling Point Of Liquid For example, for water, the boiling point is 100ºc at. When the vapour pressure equals atmospheric pressure, the liquid boils. A liquid boils at a temperature at which its vapor pressure is equal to the pressure of the gas above it. Contaminants or other nonvolatile molecules in a liquid increase its boiling point in a phenomenon called boiling point elevation.. What Happens To Boiling Point Of Liquid.