Vinegar + Naoh Equation . here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. acid and sodium hydroxide, naoh. Would the titration have required more, less or the. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. This low ph is caused by the presence of the weak acid acetic. vinegar tastes sour because it has a ph less than 7. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: demonstration of the titration of a diluted solution of vinegar.
from www.chegg.com
vinegar tastes sour because it has a ph less than 7. acid and sodium hydroxide, naoh. Would the titration have required more, less or the. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. This low ph is caused by the presence of the weak acid acetic. demonstration of the titration of a diluted solution of vinegar. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar.
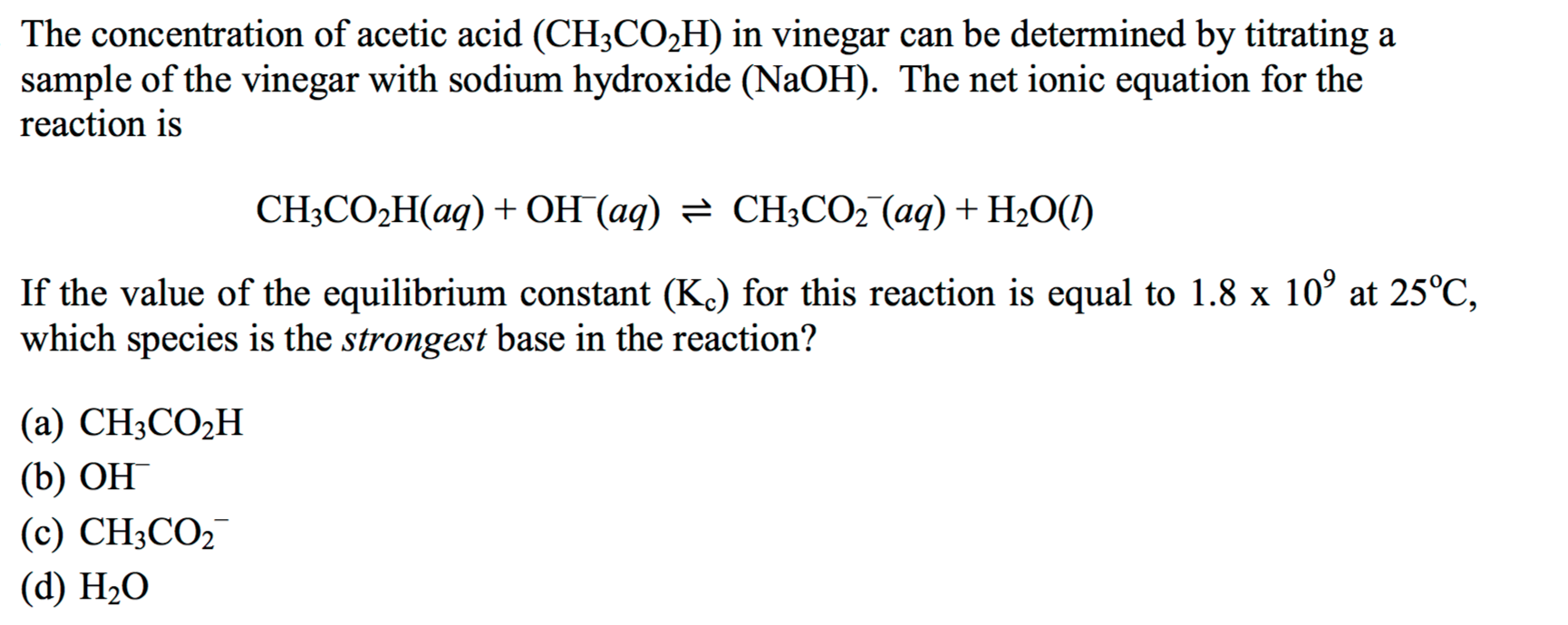
Solved The concentration of acetic acid (CH3CO2H) in vinegar
Vinegar + Naoh Equation This low ph is caused by the presence of the weak acid acetic. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: vinegar tastes sour because it has a ph less than 7. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. acid and sodium hydroxide, naoh. demonstration of the titration of a diluted solution of vinegar. Would the titration have required more, less or the. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. This low ph is caused by the presence of the weak acid acetic.
From www.numerade.com
SOLVED ANAL 466 Using Standard NaOH Solution to Analyze Vinegar Post Vinegar + Naoh Equation when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: Would the titration have required more, less or the. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED NOTE Complete both problems 1) In the standardization Vinegar + Naoh Equation This low ph is caused by the presence of the weak acid acetic. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: demonstration of the titration of a diluted solution of vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. . Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Sodium hydroxide (base) and acetic acid react in a 11 molar Vinegar + Naoh Equation suppose you added 40 ml of water to your vinegar sample instead of 20 ml. demonstration of the titration of a diluted solution of vinegar. vinegar tastes sour because it has a ph less than 7. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: Would the titration have required. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Consider the following data collected during the titration of Vinegar + Naoh Equation demonstration of the titration of a diluted solution of vinegar. This low ph is caused by the presence of the weak acid acetic. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: . Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Recording Example Problem of molarity and m/m HCHyOzaq Vinegar + Naoh Equation This low ph is caused by the presence of the weak acid acetic. vinegar tastes sour because it has a ph less than 7. Would the titration have required more, less or the. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. here, the titrant is an. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Verify your calculation Standardized NaOH (M) 0.4355 Initial Vinegar + Naoh Equation This low ph is caused by the presence of the weak acid acetic. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. acid and sodium hydroxide, naoh. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. Would the. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED A vinegar (acetic acid) solution of unknown concentration was Vinegar + Naoh Equation demonstration of the titration of a diluted solution of vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. vinegar tastes sour because it has a ph less than 7. run. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED To standardize the solution of NaOH, the student measured 0.325 Vinegar + Naoh Equation This low ph is caused by the presence of the weak acid acetic. Would the titration have required more, less or the. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. demonstration of the titration of a diluted solution of vinegar. here, the titrant is an aqueous solution of ~0.1 m. Vinegar + Naoh Equation.
From www.chegg.com
Solved Data Table 1 Titration of Vinegar with NaOH Vinegar Vinegar + Naoh Equation run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. This low ph is caused by the presence of the weak acid acetic. demonstration of the titration of a diluted solution of vinegar. vinegar tastes sour because it has a ph less than 7. when mixed, a. Vinegar + Naoh Equation.
From www.youtube.com
Find the pH of a Buffer after adding NaOH YouTube Vinegar + Naoh Equation Would the titration have required more, less or the. vinegar tastes sour because it has a ph less than 7. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. This low ph is. Vinegar + Naoh Equation.
From dxojhbucn.blob.core.windows.net
Bleach And Vinegar Reaction Equation at Herman Mathews blog Vinegar + Naoh Equation acid and sodium hydroxide, naoh. demonstration of the titration of a diluted solution of vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. This low ph is caused by the presence of the weak acid acetic. when mixed, a neutralization reaction occurs between sodium hydroxide and. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED PARALLEL DETERMINATION OF ACETIC ACID IN VINEGAR DATA Brand Vinegar + Naoh Equation here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. vinegar tastes sour because it has a ph less than 7. Would the titration have required more, less or the. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to.. Vinegar + Naoh Equation.
From www.numerade.com
Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Vinegar + Naoh Equation vinegar tastes sour because it has a ph less than 7. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. demonstration of the titration of a diluted solution of vinegar. Would the titration have required more, less or the. acid and sodium hydroxide, naoh. when mixed, a neutralization reaction. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Suppose you are titrating vinegar, which is an acetic acid Vinegar + Naoh Equation run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. demonstration of the titration of a diluted solution of vinegar. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Next, refer to the balanced chemical equation for the reaction Vinegar + Naoh Equation demonstration of the titration of a diluted solution of vinegar. This low ph is caused by the presence of the weak acid acetic. acid and sodium hydroxide, naoh. Would the titration have required more, less or the. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. when mixed, a neutralization. Vinegar + Naoh Equation.
From www.youtube.com
Equation for NaOH + H2O (Sodium hydroxide + Water) YouTube Vinegar + Naoh Equation demonstration of the titration of a diluted solution of vinegar. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: This low ph is caused by the presence of the weak acid acetic.. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Titration 1. (3 pts) The balanced equation for the titration of Vinegar + Naoh Equation demonstration of the titration of a diluted solution of vinegar. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: Would the titration have required more, less or the. This low ph is caused by the presence of the weak acid acetic. vinegar tastes sour because it has a ph less than. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Suppose you are titrating vinegar, which is an acetic acid Vinegar + Naoh Equation This low ph is caused by the presence of the weak acid acetic. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: acid and sodium hydroxide, naoh. vinegar tastes sour because it has a ph less than 7. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED The reaction of NaOH with vinegar is shown in the equation Vinegar + Naoh Equation suppose you added 40 ml of water to your vinegar sample instead of 20 ml. Would the titration have required more, less or the. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. acid and sodium hydroxide, naoh. vinegar tastes sour because it has a ph. Vinegar + Naoh Equation.
From studylib.net
The Titration of Acetic Acid in Vinegar Vinegar + Naoh Equation run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. This low ph is caused by the presence of the weak acid acetic. vinegar tastes sour because it has a. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Suppose you are titrating vinegar; which is an acetic acid Vinegar + Naoh Equation vinegar tastes sour because it has a ph less than 7. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: Would the titration have required more, less or the. This low ph. Vinegar + Naoh Equation.
From www.chegg.com
Solved The concentration of acetic acid (CH3CO2H) in vinegar Vinegar + Naoh Equation suppose you added 40 ml of water to your vinegar sample instead of 20 ml. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. vinegar tastes sour because it has a ph less than 7. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED 1) In the standardization titration of NaOH, the Molarity (M Vinegar + Naoh Equation when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: acid and sodium hydroxide, naoh. vinegar tastes sour because it has a ph less than 7. Would the titration have required more, less or the. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED 'Prelaboratory Questions A student wants to calculate the Vinegar + Naoh Equation Would the titration have required more, less or the. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. suppose you added 40 ml of water to your vinegar sample. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Texts What is the volume of NaOH (ml)? and what is the Vinegar + Naoh Equation vinegar tastes sour because it has a ph less than 7. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. demonstration of the titration of a diluted solution of vinegar. Would the titration have required more, less or the. acid and sodium hydroxide, naoh. here, the titrant is an. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Acetic acid is an important ingredient of vinegar. A sample of Vinegar + Naoh Equation acid and sodium hydroxide, naoh. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. vinegar tastes sour because it has a ph less than 7. Would the titration have required more,. Vinegar + Naoh Equation.
From gbu-taganskij.ru
Naoh Chemical Formula Utterly Stylish gbutaganskij.ru Vinegar + Naoh Equation when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: demonstration of the titration of a diluted solution of vinegar. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. This low ph is caused by the presence of the weak acid acetic. vinegar tastes sour. Vinegar + Naoh Equation.
From exoyiwzey.blob.core.windows.net
Titration Lab With Vinegar And Naoh at Edward Gross blog Vinegar + Naoh Equation when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: suppose you added 40 ml of water to your vinegar sample instead of 20 ml. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to. here, the titrant is an aqueous solution. Vinegar + Naoh Equation.
From www.studocu.com
Full PDF The Vinegar Formula Guide The Vinegar Formula Guide This one Vinegar + Naoh Equation here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. Would the titration have required more, less or the. vinegar tastes sour because it has a ph less than 7. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask until the solution changes colour from colourless to.. Vinegar + Naoh Equation.
From mathematics-chemistry.blogspot.com
Online Mathematics and Chemistry Help 10.00 mL of vinegar (mass=10.05 Vinegar + Naoh Equation This low ph is caused by the presence of the weak acid acetic. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. Would the titration have required more, less or the. suppose you added 40 ml of water to your vinegar sample instead of 20 ml. when mixed,. Vinegar + Naoh Equation.
From www.coursehero.com
[Solved] A 0.119 M NaOH solution was used to titrate a sample of Vinegar + Naoh Equation acid and sodium hydroxide, naoh. Would the titration have required more, less or the. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. This low ph is caused by the presence of the weak acid acetic. demonstration of the titration of a diluted solution of vinegar. when. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED Suppose you are titrating vinegar, which is an acetic acid Vinegar + Naoh Equation when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. demonstration of the titration of a diluted solution of vinegar. Would the titration have required more, less or the. suppose you added 40. Vinegar + Naoh Equation.
From stickerciao.es.tl
stickerciao reaction equation for acetic acid and sodium hydroxide Vinegar + Naoh Equation acid and sodium hydroxide, naoh. here, the titrant is an aqueous solution of ~0.1 m sodium hydroxide (\(\ce{naoh}\)) and the analyte is vinegar. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: demonstration of the titration of a diluted solution of vinegar. This low ph is caused by the presence. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED From this mole value of NaOH, obtain the moles of CH3COOH in Vinegar + Naoh Equation Would the titration have required more, less or the. vinegar tastes sour because it has a ph less than 7. when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: demonstration of the titration of a diluted solution of vinegar. run naoh(aq) from the burette (buret) into the conical (erlenmeyer) flask. Vinegar + Naoh Equation.
From www.numerade.com
SOLVED 1a.) Write a net ionic equation for the titration of vinegar Vinegar + Naoh Equation when mixed, a neutralization reaction occurs between sodium hydroxide and the acetic acid in vinegar: demonstration of the titration of a diluted solution of vinegar. This low ph is caused by the presence of the weak acid acetic. vinegar tastes sour because it has a ph less than 7. Would the titration have required more, less or. Vinegar + Naoh Equation.