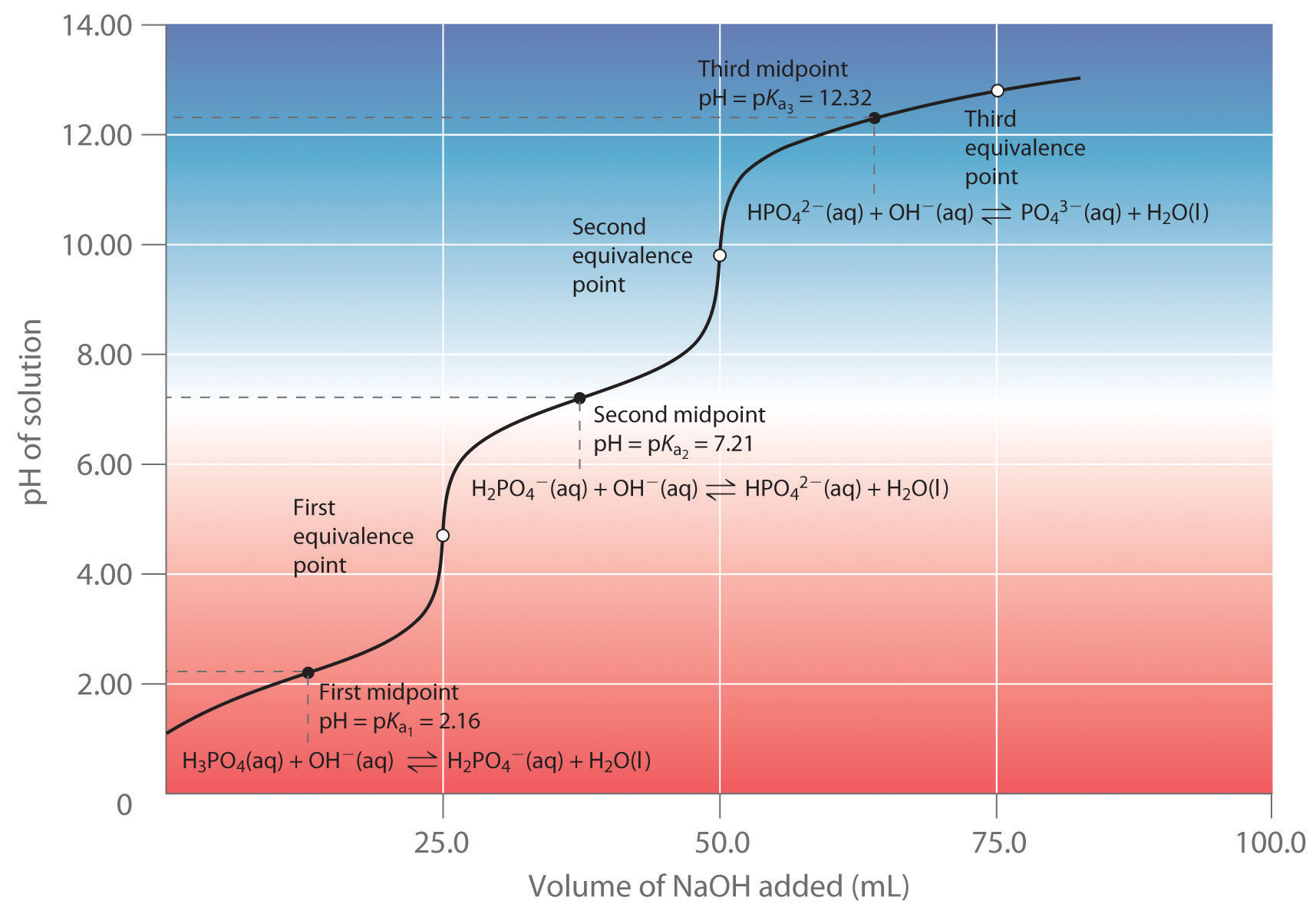
Titration Curves In Chemistry . A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The shape of the graph produced is called a titration curve. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. It provides valuable information about the reaction under study and helps. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The equivalence point of a titration.
from chem.libretexts.org
During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The shape of the graph produced is called a titration curve. Both equivalence points are visible. The figure below shows two different examples of a strong. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant.
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts
Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The shape of the graph produced is called a titration curve. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. The equivalence point of a titration. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The figure below shows two different examples of a strong.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curves In Chemistry The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph.. Titration Curves In Chemistry.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Curves In Chemistry The figure below shows two different examples of a strong. The equivalence point of a titration. The shape of the graph produced is called a titration curve. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. A titration curve is a graphical representation of. Titration Curves In Chemistry.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. The shape of the graph produced is called a titration curve. A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. The equivalence point of a titration.. Titration Curves In Chemistry.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. A typical titration curve of a diprotic acid, oxalic acid, titrated with a. Titration Curves In Chemistry.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Titration Curves In Chemistry The shape of the graph produced is called a titration curve. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. During a titration, ph can be plotted against the volume of acid added to a basic solution. Titration Curves In Chemistry.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Curves In Chemistry During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The shape of the graph produced is called a titration curve. It provides valuable information about the reaction under study and helps. The figure below shows two different examples of a strong. A titration curve. Titration Curves In Chemistry.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. The shape of the graph produced is called a titration curve. A typical titration curve of a diprotic acid, oxalic. Titration Curves In Chemistry.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curves In Chemistry The figure below shows two different examples of a strong. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. A typical titration curve of a diprotic. Titration Curves In Chemistry.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curves In Chemistry Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of the ph of. Titration Curves In Chemistry.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Titration Curves In Chemistry During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. A titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of. Titration Curves In Chemistry.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curves In Chemistry The shape of the graph produced is called a titration curve. A titration curve is a graphical representation of the ph of a solution during a titration. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. Both equivalence points are visible. During a titration, ph can be plotted against the volume. Titration Curves In Chemistry.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curves In Chemistry The figure below shows two different examples of a strong. It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. Both equivalence points are visible. The titration curve is a graphical representation of the ph or other. Titration Curves In Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. The shape of the graph produced is called a titration curve. The titration curve is a graphical representation of the ph or. Titration Curves In Chemistry.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curves In Chemistry The shape of the graph produced is called a titration curve. It provides valuable information about the reaction under study and helps. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The figure below shows two different examples of a strong. A titration curve. Titration Curves In Chemistry.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Curves In Chemistry It provides valuable information about the reaction under study and helps. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. During a titration, ph can be plotted against the volume of. Titration Curves In Chemistry.
From www.youtube.com
AP Chem unit 8.5 Titration Curves YouTube Titration Curves In Chemistry The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. A titration curve is. Titration Curves In Chemistry.
From edurev.in
Graphical Representation of Titration Curves Chemistry for JAMB PDF Titration Curves In Chemistry During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The figure below shows two different examples of a strong. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of. Titration Curves In Chemistry.
From www.youtube.com
Titration Curves for High School Chemistry YouTube Titration Curves In Chemistry The figure below shows two different examples of a strong. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. It. Titration Curves In Chemistry.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. The shape of the graph produced is called a titration curve. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. A titration curve is a graphical representation of. Titration Curves In Chemistry.
From crunchchemistry.co.uk
How to explain the shape of a titration curve Crunch Chemistry Titration Curves In Chemistry The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. The equivalence point of a titration. Both equivalence points are visible. The titration curve is a graphical representation of the ph or other property changes during a titration. Titration Curves In Chemistry.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. The shape of the graph produced is called a titration curve. The. Titration Curves In Chemistry.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curves In Chemistry The titration curve is a graphical representation of the ph or other property changes during a titration experiment. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The figure below shows two different examples of a strong. The equivalence point of a titration. A titration curve is a graphical representation of the. Titration Curves In Chemistry.
From www.afterskool.com.sg
HL/ H2 Chemistry 101 Titration Curves Summary Guide — AfterSkool Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. During a titration, ph can be plotted against the volume of acid added to a basic solution. Titration Curves In Chemistry.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Titration Curves In Chemistry The shape of the graph produced is called a titration curve. Both equivalence points are visible. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. It provides valuable information about the reaction under study and helps. The titration curve is a graphical representation of the ph or. Titration Curves In Chemistry.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Curves In Chemistry The figure below shows two different examples of a strong. The shape of the graph produced is called a titration curve. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. It provides valuable information about the reaction under study and helps. Both equivalence points are visible. A titration curve is a graphical. Titration Curves In Chemistry.
From www.youtube.com
TRU Chemistry labs How To Plot a Titration Curve YouTube Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. The equivalence point of a titration. Both equivalence points are visible. A titration curve is a graphical representation of. Titration Curves In Chemistry.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curves In Chemistry The figure below shows two different examples of a strong. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. During a titration, ph can be plotted against the volume of acid added to a basic solution (or the other way round!) in a graph. It provides valuable. Titration Curves In Chemistry.
From www.youtube.com
Acid Base Titration Curves Simplified YouTube Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. The titration curve is a graphical representation of the ph or other property changes during a. Titration Curves In Chemistry.
From mungfali.com
Buffer Region On Titration Curve Titration Curves In Chemistry The shape of the graph produced is called a titration curve. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. The titration curve is a. Titration Curves In Chemistry.
From general.chemistrysteps.com
Titration of a Polyprotic Acids Chemistry Steps Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. Titration Curves In Chemistry.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Curves In Chemistry It provides valuable information about the reaction under study and helps. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different examples of a strong. During a titration, ph can be plotted against the volume of acid added to a basic solution. Titration Curves In Chemistry.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curves In Chemistry A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. The shape of the graph produced is called a titration curve. The titration curve is a graphical representation of the ph or other property changes during a titration experiment. During a titration, ph can be plotted against the volume of acid added to. Titration Curves In Chemistry.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Titration Curves In Chemistry A titration curve is a graphical representation of the ph of a solution during a titration. The equivalence point of a titration. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. The figure below shows two different examples of a strong. The shape of the graph produced. Titration Curves In Chemistry.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curves In Chemistry The shape of the graph produced is called a titration curve. A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. A titration curve is a graphical representation of the ph of a solution as a function of the volume of added titrant. The equivalence point of a titration. The titration curve is. Titration Curves In Chemistry.
From www.purechemistry.org
Precipitation Titration Purechemistry Titration Curves In Chemistry A typical titration curve of a diprotic acid, oxalic acid, titrated with a strong base, sodium hydroxide. It provides valuable information about the reaction under study and helps. The shape of the graph produced is called a titration curve. A titration curve is a graphical representation of the ph of a solution during a titration. Both equivalence points are visible.. Titration Curves In Chemistry.