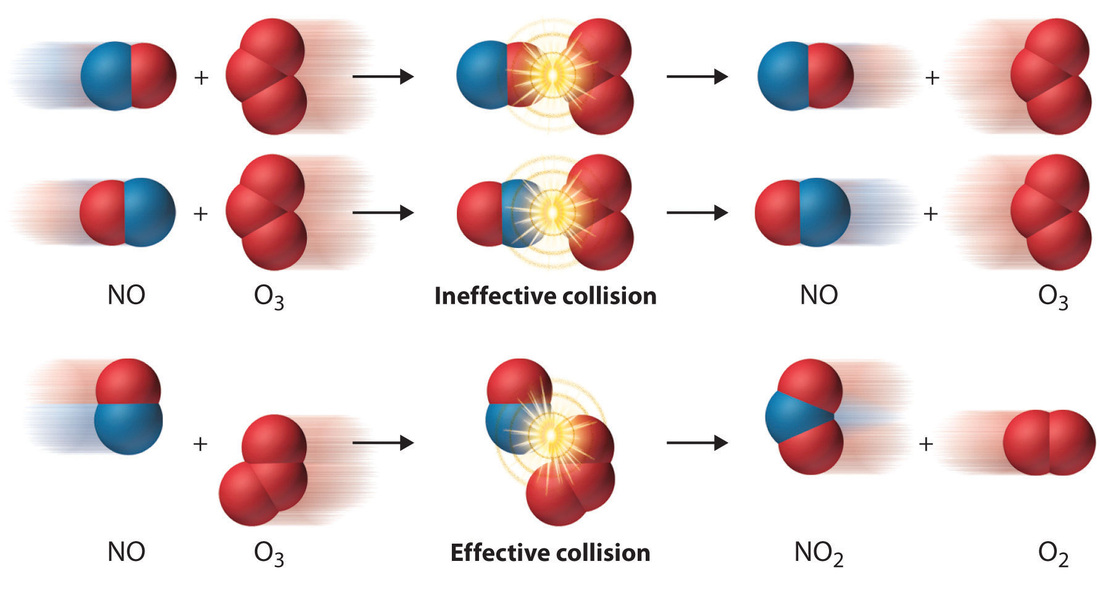
What Are The Example Of Reaction Rate . For example, wood combustion has a high reaction rate since the. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. It is expressed as the concentration of the reactants consumed or products formed per unit time. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. The rate of reaction is proportional to the number of collisions over time; Increasing the concentration of either reactant increases the. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. The rate of reaction is the change in the amount of a reactant or product per unit time. For reactions involving one or. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. Reaction rates are therefore determined by measuring the time.
from mrtremblaycambridge.weebly.com
Increasing the concentration of either reactant increases the. For reactions involving one or. It is expressed as the concentration of the reactants consumed or products formed per unit time. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. The rate of reaction is the change in the amount of a reactant or product per unit time. The rate of reaction is proportional to the number of collisions over time; It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. Reaction rates are therefore determined by measuring the time.
C12. Rates of reaction Mr. Tremblay's Class Site
What Are The Example Of Reaction Rate For example, wood combustion has a high reaction rate since the. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. For reactions involving one or. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. For example, wood combustion has a high reaction rate since the. The rate of reaction is proportional to the number of collisions over time; Reaction rates are therefore determined by measuring the time. It is expressed as the concentration of the reactants consumed or products formed per unit time. Increasing the concentration of either reactant increases the. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. The rate of reaction is the change in the amount of a reactant or product per unit time.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6516140 What Are The Example Of Reaction Rate Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. For example, wood combustion has a high reaction rate since the. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. It is expressed as the concentration. What Are The Example Of Reaction Rate.
From www.expii.com
Factors Affecting Reaction Rate — Overview & Examples Expii What Are The Example Of Reaction Rate For example, wood combustion has a high reaction rate since the. Reaction rates are therefore determined by measuring the time. The rate of reaction is proportional to the number of collisions over time; The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. Reaction rate, in chemistry, the speed at which a chemical reaction. What Are The Example Of Reaction Rate.
From sciencenotes.org
Factors That Affect Reaction Rate Chemical What Are The Example Of Reaction Rate As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. It is often expressed in terms of either the concentration (amount per unit volume) of a product. What Are The Example Of Reaction Rate.
From biology.reachingfordreams.com
Reaction rates Biology What Are The Example Of Reaction Rate The rate of reaction is proportional to the number of collisions over time; For example, wood combustion has a high reaction rate since the. For reactions involving one or. The rate of reaction is the change in the amount of a reactant or product per unit time. Increasing the concentration of either reactant increases the. It is expressed as the. What Are The Example Of Reaction Rate.
From www.tes.com
Rates of reactions (Chemistry) Teaching Resources What Are The Example Of Reaction Rate For reactions involving one or. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. For example, wood combustion has a high reaction rate since the. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. As per the general definition, the speed with which a reaction takes place is referred to. What Are The Example Of Reaction Rate.
From www.slideshare.net
Rates of reaction What Are The Example Of Reaction Rate The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The rate of reaction is the change in the amount of a reactant or product per unit time. The rate of reaction is proportional to the number of collisions over time; Rates of reactions that consume or produce gaseous substances, for example, are conveniently. What Are The Example Of Reaction Rate.
From www.youtube.com
Reaction Rates and Stoichiometry Chemistry Tutorial YouTube What Are The Example Of Reaction Rate Reaction rates are therefore determined by measuring the time. It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction is the change in the amount of a reactant or product per unit time. As per the general definition, the speed with which a reaction takes place is referred to as. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Reaction Rates (Chapter 13) PowerPoint Presentation, free What Are The Example Of Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. For reactions involving one or. For example, wood combustion has a high reaction rate since the. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. The rate of reaction is. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Definition of Reaction Rate PowerPoint Presentation, free What Are The Example Of Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. The rate of reaction is proportional to the number of collisions over time; Increasing the concentration of either reactant increases the. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. For reactions involving one or.. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 What Are The Example Of Reaction Rate Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. The rate of reaction is proportional to the number of collisions over time; As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. The reaction rate or rate of reaction is the speed at which a. What Are The Example Of Reaction Rate.
From www.onlinebiologynotes.com
Rate of enzyme reactions and factor affecting the rate of enzyme What Are The Example Of Reaction Rate The rate of reaction is proportional to the number of collisions over time; Increasing the concentration of either reactant increases the. For reactions involving one or. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. The rate of reaction is the change in the amount of a. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 What Are The Example Of Reaction Rate The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. Reaction rates are therefore determined by measuring the time. The rate of reaction is proportional to the number of collisions over time;. What Are The Example Of Reaction Rate.
From www.sliderbase.com
Rates of reactions Presentation Chemistry What Are The Example Of Reaction Rate Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. For example, wood combustion. What Are The Example Of Reaction Rate.
From en.ppt-online.org
Rates of reaction online presentation What Are The Example Of Reaction Rate The rate of reaction is proportional to the number of collisions over time; As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. It is expressed as the concentration. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Rates of Reaction and Chemical Equilibrium PowerPoint What Are The Example Of Reaction Rate It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. The rate of reaction is the change in the amount of a reactant or product per unit time. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. The rate of reaction is proportional to. What Are The Example Of Reaction Rate.
From www.sasadoctor.com
Rate of reaction chemistry igcse What Are The Example Of Reaction Rate It is expressed as the concentration of the reactants consumed or products formed per unit time. For reactions involving one or. For example, wood combustion has a high reaction rate since the. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. Increasing the concentration of either reactant increases the. As. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Reaction Rate and Equilibrium PowerPoint Presentation, free What Are The Example Of Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. Increasing the concentration of either reactant increases the. Reaction rates are therefore determined by measuring the time. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. Reaction rate, in chemistry, the speed. What Are The Example Of Reaction Rate.
From www.youtube.com
Chemical 2 Average Rate of Reaction Instantaneous Rate What Are The Example Of Reaction Rate For reactions involving one or. The rate of reaction is the change in the amount of a reactant or product per unit time. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. The rate of reaction is proportional to the number of collisions over time; For example,. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID1785599 What Are The Example Of Reaction Rate The rate of reaction is proportional to the number of collisions over time; For example, wood combustion has a high reaction rate since the. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. It is expressed as the concentration of the reactants consumed or products formed per. What Are The Example Of Reaction Rate.
From www.expii.com
Rate of Reaction (Enzymes) — Role & Importance Expii What Are The Example Of Reaction Rate Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. Increasing the concentration of either reactant increases the. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is.. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Rate of reaction definition PowerPoint Presentation, free What Are The Example Of Reaction Rate It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. The rate of reaction is the change in the amount of a reactant or product per unit time. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. For reactions. What Are The Example Of Reaction Rate.
From www.sasadoctor.com
Rate of reaction chemistry igcse What Are The Example Of Reaction Rate It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. For reactions involving one or. The rate of reaction is the change in the amount of a reactant or product per unit time. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in. What Are The Example Of Reaction Rate.
From www.sasadoctor.com
Rate of reaction chemistry igcse What Are The Example Of Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. For reactions involving one or. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. It is expressed as the concentration of the reactants consumed or products formed per unit time. As per the general definition,. What Are The Example Of Reaction Rate.
From studylib.net
Chemical reaction rates What Are The Example Of Reaction Rate It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. It is expressed as the concentration of the reactants consumed or products formed per unit time. The rate of reaction is the change in the amount of a reactant or product per unit time. The reaction rate or rate of reaction. What Are The Example Of Reaction Rate.
From study.com
How to Determine the Order of Reaction by Comparing Initial Rates of What Are The Example Of Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. The rate of reaction is proportional to the number of collisions over time; The reaction rate or rate of reaction. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 What Are The Example Of Reaction Rate Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. It is expressed as the concentration of the reactants consumed or products formed per unit time. Increasing. What Are The Example Of Reaction Rate.
From studylib.net
The Rate of Chemical Reactions What Are The Example Of Reaction Rate The rate of reaction is the change in the amount of a reactant or product per unit time. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. As per. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Rate of Reaction PowerPoint Presentation, free download ID2483456 What Are The Example Of Reaction Rate Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. It is expressed as the concentration of the. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Reaction Rates PowerPoint Presentation, free download ID6090305 What Are The Example Of Reaction Rate It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. The rate of reaction is proportional to the number of collisions over time; For example, wood combustion has a high reaction rate since the. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes. What Are The Example Of Reaction Rate.
From www.youtube.com
Calculating Rates of Reaction (2016) IB Biology YouTube What Are The Example Of Reaction Rate Reaction rates are therefore determined by measuring the time. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. Increasing the concentration of either reactant increases the. For example, wood combustion has a high reaction rate since the. It is expressed as the concentration of the reactants consumed or. What Are The Example Of Reaction Rate.
From mrtremblaycambridge.weebly.com
C12. Rates of reaction Mr. Tremblay's Class Site What Are The Example Of Reaction Rate For reactions involving one or. It is expressed as the concentration of the reactants consumed or products formed per unit time. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. For example, wood combustion has a high reaction rate since the. The rate of reaction is proportional to the number. What Are The Example Of Reaction Rate.
From www.slideserve.com
PPT Reaction Rates & Equilibrium PowerPoint Presentation, free What Are The Example Of Reaction Rate Reaction rates are therefore determined by measuring the time. Reaction rate, in chemistry, the speed at which a chemical reaction proceeds. For example, wood combustion has a high reaction rate since the. Increasing the concentration of either reactant increases the. It is often expressed in terms of either the concentration (amount per unit volume) of a product that is. As. What Are The Example Of Reaction Rate.
From hubpages.com
Chemical Reactions and Chemical Equations Owlcation What Are The Example Of Reaction Rate Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined by measuring changes in volume or pressure. For example, wood combustion has a high reaction rate since the. Reaction rates are therefore determined by measuring the time. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. For reactions involving. What Are The Example Of Reaction Rate.
From www.slideshare.net
Reaction Rate Graph Tutorial 2 What Are The Example Of Reaction Rate Reaction rates are therefore determined by measuring the time. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. For example, wood combustion has a high reaction rate since the. The rate of reaction is proportional to the number of collisions over time; The rate of reaction is the change in the amount of. What Are The Example Of Reaction Rate.
From www.slideshare.net
Rates of chemical reactions What Are The Example Of Reaction Rate As per the general definition, the speed with which a reaction takes place is referred to as the rate of a reaction. The reaction rate or rate of reaction is the speed at which a chemical reaction proceeds. Increasing the concentration of either reactant increases the. Rates of reactions that consume or produce gaseous substances, for example, are conveniently determined. What Are The Example Of Reaction Rate.