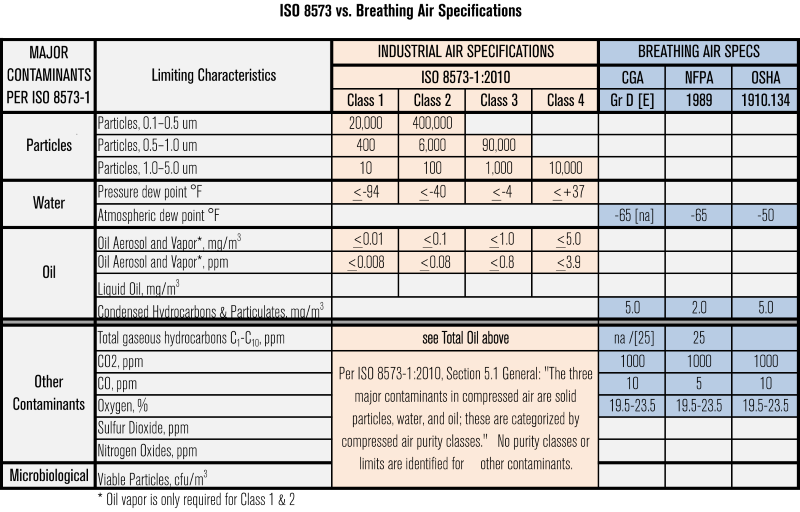
Compressed Air Qualification Guidelines . In addition to the main text, appendices on some validation and. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. 1.2 these guidelines cover the general principles of qualification and validation. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. Often referred to as the 4th utility after electricity, water, and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. Iso 8573 is the group of international standards relating to the. A guide to benchmarking performance with iso 12500, and. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed.
from cedzbxje.blob.core.windows.net
Iso 8573 is the group of international standards relating to the. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. In addition to the main text, appendices on some validation and. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. A guide to benchmarking performance with iso 12500, and. Often referred to as the 4th utility after electricity, water, and. 1.2 these guidelines cover the general principles of qualification and validation.
Fda Requirements For Compressed Air at Judy Rios blog
Compressed Air Qualification Guidelines The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. Often referred to as the 4th utility after electricity, water, and. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. A guide to benchmarking performance with iso 12500, and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. Iso 8573 is the group of international standards relating to the. 1.2 these guidelines cover the general principles of qualification and validation. In addition to the main text, appendices on some validation and. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with.
From www.airchecklab.com
How to Designate ISO 85731 Purity Classes Trace Analytics, the Compressed Air Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. In addition to the main text, appendices on some validation and. Often referred to as the 4th utility after electricity, water, and. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. A guide. Compressed Air Qualification Guidelines.
From www.pharmaspecialists.com
ISO 85731 Guideline for Compressed Air Compressed Air Qualification Guidelines As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. 1.2 these guidelines cover the general principles of qualification and validation. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the. Compressed Air Qualification Guidelines.
From blog.exair.com
Compressed Air Quality and ISO 85731 Purity Classes Compressed Air Qualification Guidelines The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. Iso 8573 is the group of international standards relating to the. As. Compressed Air Qualification Guidelines.
From studylib.net
Compressed Air Leak guidelines Compressed Air Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. Often referred to as the 4th utility after electricity, water, and. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. The ispe good practice guide for process gases asserts that. Compressed Air Qualification Guidelines.
From nationalsafety.wordpress.com
Top 10 Compressed Air Safety Guidelines (Infographic) Nationalsafety Compressed Air Qualification Guidelines The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. In addition to the main text, appendices on some validation and. Iso 8573 is the group of international standards relating to the. 1.2 these guidelines cover the general principles of qualification and validation. Often referred to as the 4th. Compressed Air Qualification Guidelines.
From www.scribd.com
Water Qualification and Compressed Air Qualification PDF Membrane Ph Compressed Air Qualification Guidelines In addition to the main text, appendices on some validation and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. Iso 8573 is the group of international standards relating to the. A guide to benchmarking performance with iso 12500, and. The compressed air testing for the presence of. Compressed Air Qualification Guidelines.
From www.gosuburban.com
How to Determine Poor Air Quality in Compressed Air Compressed Air Qualification Guidelines Iso 8573 is the group of international standards relating to the. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. 1.2 these guidelines cover the general principles of qualification and validation. Often referred to as the 4th utility after electricity, water,. Compressed Air Qualification Guidelines.
From www.airchecklab.com
Compressed Air System Risk Assessment Do I Need to Test? Trace Compressed Air Qualification Guidelines In addition to the main text, appendices on some validation and. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. 1.2 these guidelines cover the general principles of qualification and validation. As a voluntary code of practice, bcas food and beverage grade compressed air. Compressed Air Qualification Guidelines.
From www.tal.sg
WSH Guidelines on Compressed Air Works Prevention of Compressed Air Compressed Air Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. Iso 8573 is the group of international standards relating to the. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. The ispe good practice guide for process gases asserts that compressed air usage. Compressed Air Qualification Guidelines.
From nl.pinterest.com
Top 12 Compressed Air Safety Guidelines [Infographic] Compressed air Compressed Air Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. Often referred to as the 4th utility after electricity, water, and. Iso 8573 is the group of international standards relating to. Compressed Air Qualification Guidelines.
From flairpharma.com
Compressed Air Qualification » Flair Pharma The Knowledge Kit. Compressed Air Qualification Guidelines As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. Often referred to as the 4th utility after electricity, water, and. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that. Compressed Air Qualification Guidelines.
From pharmabeginers.com
HVAC System SOP for Qualification Pharma Beginners Compressed Air Qualification Guidelines This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. 1.2 these guidelines cover the general principles. Compressed Air Qualification Guidelines.
From www.bcas.org.uk
Food & Beverage Grade Compressed Air Best Practice Guideline Compressed Air Qualification Guidelines In addition to the main text, appendices on some validation and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. As. Compressed Air Qualification Guidelines.
From www.youtube.com
Compressed air Qualification Compressed air Validation Compressed Air Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. A guide to benchmarking performance with iso 12500, and. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. This part of iso 8573 specifies purity classes of compressed air with respect. Compressed Air Qualification Guidelines.
From globalehs.co.in
Air Compressor Inspection Checklist Compressed Air Qualification Guidelines As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. Often referred to as the. Compressed Air Qualification Guidelines.
From www.scribd.com
Compressed Air Guidelines Gas Compressor Valve Compressed Air Qualification Guidelines Iso 8573 is the group of international standards relating to the. Often referred to as the 4th utility after electricity, water, and. A guide to benchmarking performance with iso 12500, and. In addition to the main text, appendices on some validation and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be. Compressed Air Qualification Guidelines.
From www.scribd.com
Compressed Air Validation PDF Gases Energy Technology Compressed Air Qualification Guidelines A guide to benchmarking performance with iso 12500, and. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. Often referred. Compressed Air Qualification Guidelines.
From dokumen.tips
(PDF) Compressed Air Guidelines DOKUMEN.TIPS Compressed Air Qualification Guidelines This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. Iso 8573 is the group of international standards relating to the. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. In addition to. Compressed Air Qualification Guidelines.
From www.scribd.com
Compressed Air Qualification Gaps Standard Requirement 1) Dew Point Compressed Air Qualification Guidelines The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. Iso 8573 is the group of international standards relating to the. A guide to benchmarking performance with iso 12500, and. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent. Compressed Air Qualification Guidelines.
From www.industrialair.co.nz
Compressed air quality standards Compressed Air Qualification Guidelines In addition to the main text, appendices on some validation and. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming. Compressed Air Qualification Guidelines.
From about.ita-aites.org
Guidelines for good working practice in high pressure compressed air Compressed Air Qualification Guidelines This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. Iso 8573 is the group of international standards. Compressed Air Qualification Guidelines.
From www.milessupply.com
Chart for Compressed Air and Sandblasting Stone Accessories Compressed Air Qualification Guidelines Iso 8573 is the group of international standards relating to the. In addition to the main text, appendices on some validation and. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. The ispe good practice guide for process gases asserts that. Compressed Air Qualification Guidelines.
From www.slideserve.com
PPT Heating, Ventilation and AirConditioning (HVAC) Part 3 Compressed Air Qualification Guidelines The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. A guide to benchmarking performance with iso 12500, and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. This part of. Compressed Air Qualification Guidelines.
From go.gale.com
Design and specification of a compressed air system a practical Compressed Air Qualification Guidelines This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. Iso 8573 is the group of international standards relating to the. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct. Compressed Air Qualification Guidelines.
From www.bcas.org.uk
Pressure and Leak Testing of Compressed Air Systems Best Practice Compressed Air Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. Iso 8573 is the group of international standards relating to the. Often referred to as the 4th utility after electricity, water,. Compressed Air Qualification Guidelines.
From www.scribd.com
Requirements for Compressed Air in the Pharmaceutical Industry Gases Compressed Air Qualification Guidelines Often referred to as the 4th utility after electricity, water, and. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that. Compressed Air Qualification Guidelines.
From www.industrialoutpost.com
Compressed Air Requirements Chart Industrial Outpost The Official Compressed Air Qualification Guidelines The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. 1.2 these guidelines cover the general principles of qualification and validation. In addition to the main text, appendices on some validation and. Iso 8573 is the group of international standards relating to the.. Compressed Air Qualification Guidelines.
From compressedairservices.com
Compressed Air Requirements By Compressed Air Services Compressed Air Qualification Guidelines Often referred to as the 4th utility after electricity, water, and. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. A guide to benchmarking performance with iso 12500, and. As a voluntary code of practice, bcas food and beverage grade compressed air best practice. Compressed Air Qualification Guidelines.
From www.academia.edu
(PDF) Qualification Compressed Air System Compressed Air Qualification Guidelines This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. 1.2 these guidelines cover the general principles. Compressed Air Qualification Guidelines.
From www.youtube.com
Compressed air system Compressed air flow diagram Compressed Air Compressed Air Qualification Guidelines Iso 8573 is the group of international standards relating to the. 1.2 these guidelines cover the general principles of qualification and validation. In addition to the main text, appendices on some validation and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. Often referred to as the 4th. Compressed Air Qualification Guidelines.
From pharmaguidehub.com
QUALIFICATION OF PROTOCOL CUM REPORT OF COMPRESSED AIR GENERATION AND Compressed Air Qualification Guidelines As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. In addition to the main text, appendices on some validation and.. Compressed Air Qualification Guidelines.
From www.scribd.com
Qualification of Compressed Air Protocol PDF Piston Oxide Compressed Air Qualification Guidelines Iso 8573 is the group of international standards relating to the. As a voluntary code of practice, bcas food and beverage grade compressed air best practice guideline 102 stipulates compressed air that is in direct contact with the product. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the. Compressed Air Qualification Guidelines.
From jhfoster.com
Ensure Compressed Air Purity Levels Using ISO JHFOSTER Compressed Air Qualification Guidelines 1.2 these guidelines cover the general principles of qualification and validation. In addition to the main text, appendices on some validation and. This part of iso 8573 specifies purity classes of compressed air with respect to particles, water and oil, independent of the location in the compressed. A guide to benchmarking performance with iso 12500, and. The ispe good practice. Compressed Air Qualification Guidelines.
From in.pinterest.com
Safety Tips for usage of Compressed Air Equipment Compressed Air Qualification Guidelines Often referred to as the 4th utility after electricity, water, and. 1.2 these guidelines cover the general principles of qualification and validation. The compressed air testing for the presence of moisture, the presence of oil and total viable aerobic count and pathogens that is directly coming in contact with. The ispe good practice guide for process gases asserts that compressed. Compressed Air Qualification Guidelines.
From cedzbxje.blob.core.windows.net
Fda Requirements For Compressed Air at Judy Rios blog Compressed Air Qualification Guidelines Iso 8573 is the group of international standards relating to the. In addition to the main text, appendices on some validation and. Often referred to as the 4th utility after electricity, water, and. The ispe good practice guide for process gases asserts that compressed air usage in pharmaceutical manufacturing should be free from contaminants. 1.2 these guidelines cover the general. Compressed Air Qualification Guidelines.