Bleach Redox Equation . Write a balanced equation for the reaction of. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. write a balanced equation for the reaction of bleach with potassium iodide. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Oxidation is most generally defined as. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. h2o2 æ 1⁄2 o2 + h2o.
from www.chegg.com
write a balanced equation for the reaction of bleach with potassium iodide. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. h2o2 æ 1⁄2 o2 + h2o. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. Oxidation is most generally defined as. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. Write a balanced equation for the reaction of. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample.
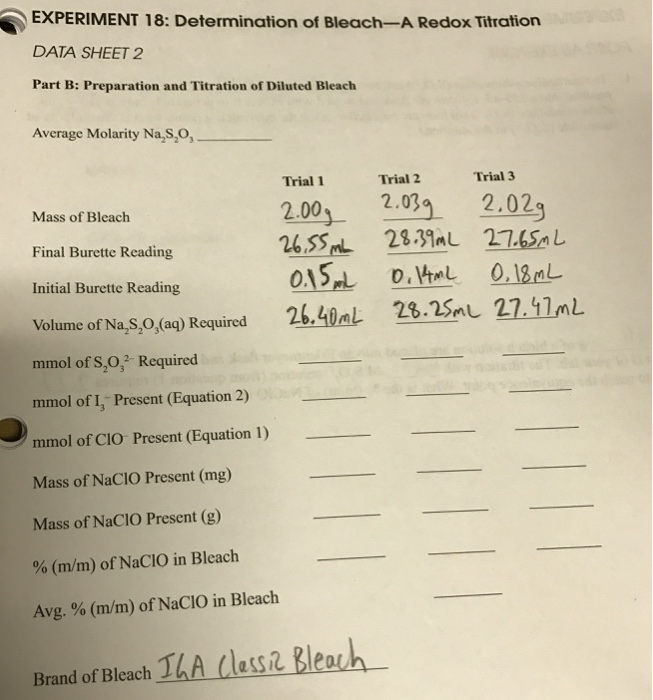
Solved EXPERIMENT 18 Determination Of BleachA Redox Tit...
Bleach Redox Equation h2o2 æ 1⁄2 o2 + h2o. write a balanced equation for the reaction of bleach with potassium iodide. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. h2o2 æ 1⁄2 o2 + h2o. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. Write a balanced equation for the reaction of. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Oxidation is most generally defined as. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood.
From www.numerade.com
In this section, write the redox equation for the… Bleach Redox Equation 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. write a balanced equation for the reaction of bleach with potassium iodide. Write a balanced equation for the reaction of. Oxidation is most generally defined as. h2o2 æ. Bleach Redox Equation.
From www.youtube.com
Bleach and Hydrogen Peroxide Reaction + Balanced Equation YouTube Bleach Redox Equation Write a balanced equation for the reaction of. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. write a balanced equation for the reaction of bleach with potassium iodide. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. commercial bleaches are created by bubbling chlorine gas. Bleach Redox Equation.
From www.slideserve.com
PPT Redox Titration OF bleach PowerPoint Presentation, free download Bleach Redox Equation sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. h2o2 æ 1⁄2 o2 + h2o. Write a balanced equation for the reaction of. write a balanced equation for the reaction of bleach with. Bleach Redox Equation.
From www.youtube.com
CHEM 222 Bleach Oxidation of Alcohols YouTube Bleach Redox Equation write a balanced equation for the reaction of bleach with potassium iodide. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. h2o2 æ 1⁄2 o2 + h2o. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. commercial bleaches are created by. Bleach Redox Equation.
From studylib.net
Redox Titration Analysis of Commercial Bleach Bleach Redox Equation write a balanced equation for the reaction of bleach with potassium iodide. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample.. Bleach Redox Equation.
From chemistryfromscratch.org
V3.9 Bleach Redox Equation a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. write a balanced equation for the reaction of bleach with potassium. Bleach Redox Equation.
From www.vrogue.co
Balancing Redox Part 2 Determining Redox Reaction Ide vrogue.co Bleach Redox Equation Write a balanced equation for the reaction of. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Oxidation is most generally defined as. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called. Bleach Redox Equation.
From chemistnotes.com
How to Balance Redox Equations Using a Redox Reaction Calculator Bleach Redox Equation h2o2 æ 1⁄2 o2 + h2o. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. ordinary household bleach, sodium hypochlorite. Bleach Redox Equation.
From www.tessshebaylo.com
Balanced Chemical Equation For Hydrogen Peroxide And Bleach Tessshebaylo Bleach Redox Equation a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Oxidation is most generally defined as. Write a balanced equation for the reaction of. write a balanced equation for the reaction of bleach with potassium iodide. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. sodium hypochlorite. Bleach Redox Equation.
From www.thesciencehive.co.uk
The Halogens* — the science sauce Bleach Redox Equation h2o2 æ 1⁄2 o2 + h2o. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. commercial bleaches are created by. Bleach Redox Equation.
From www.docsity.com
Bleach Redox Iyon Postlab 8 Introduction to Chemical Practice Bleach Redox Equation 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. write a balanced equation for the reaction of bleach with potassium iodide. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. h2o2 æ. Bleach Redox Equation.
From www.chegg.com
Solved EXPERIMENT 18 Determination Of BleachA Redox Tit... Bleach Redox Equation h2o2 æ 1⁄2 o2 + h2o. Write a balanced equation for the reaction of. Oxidation is most generally defined as. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. a redox titration is conducted to determine the. Bleach Redox Equation.
From www.slideserve.com
PPT Redox Titration OF bleach PowerPoint Presentation, free download Bleach Redox Equation sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. commercial bleaches are created by bubbling chlorine gas. Bleach Redox Equation.
From www.chegg.com
Solved You will need some bleach to perform a REDOX reaction Bleach Redox Equation write a balanced equation for the reaction of bleach with potassium iodide. h2o2 æ 1⁄2 o2 + h2o. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. Oxidation is most generally defined as. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. ordinary household bleach,. Bleach Redox Equation.
From www.slideserve.com
PPT Redox Reactions PowerPoint Presentation ID3872488 Bleach Redox Equation write a balanced equation for the reaction of bleach with potassium iodide. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide. Bleach Redox Equation.
From sites.google.com
Oxidation Numbers Chemistry Hybrid Bleach Redox Equation Write a balanced equation for the reaction of. Oxidation is most generally defined as. write a balanced equation for the reaction of bleach with potassium iodide. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. sodium hypochlorite is an inexpensive,. Bleach Redox Equation.
From keplarllp.com
🎉 Analysis of commercial bleach. Redox Titration of Hypochlorite in Bleach Redox Equation Write a balanced equation for the reaction of. h2o2 æ 1⁄2 o2 + h2o. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. Oxidation is most generally defined as. ordinary household bleach, sodium hypochlorite (naclo), acts. Bleach Redox Equation.
From www.chegg.com
EXPERIMENT 18 Determination of Bleach A Redox Bleach Redox Equation sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. Oxidation is most generally defined as. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. 1) during this. Bleach Redox Equation.
From www.chegg.com
Solved Given the mechanism for bleach's oxidation of Bleach Redox Equation write a balanced equation for the reaction of bleach with potassium iodide. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. h2o2 æ 1⁄2 o2 + h2o. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Write a balanced equation for the reaction of. Oxidation. Bleach Redox Equation.
From www.youtube.com
Making Bleach AS Chemistry YouTube Bleach Redox Equation h2o2 æ 1⁄2 o2 + h2o. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. Oxidation is most generally defined as. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample.. Bleach Redox Equation.
From chubbyrevision.weebly.com
Chem/Redox Chubby Revision AS Level Bleach Redox Equation a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. h2o2 æ 1⁄2 o2 + h2o. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. write a balanced equation for. Bleach Redox Equation.
From www.slideserve.com
PPT Redox Titration OF bleach PowerPoint Presentation, free download Bleach Redox Equation ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Write a balanced equation for the reaction of. write a balanced equation for the reaction of bleach with potassium iodide. sodium hypochlorite is an. Bleach Redox Equation.
From www.slideshare.net
Redox Bleach Redox Equation ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Write a balanced equation for the reaction of. write a balanced equation for the reaction of bleach with potassium iodide. h2o2 æ 1⁄2 o2 + h2o. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. sodium. Bleach Redox Equation.
From www.youtube.com
Balancing redox equation Acidic & Basic (Cl2 = Cl^ + ClO2) Dr K Bleach Redox Equation Oxidation is most generally defined as. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. commercial bleaches are created by bubbling. Bleach Redox Equation.
From slidetodoc.com
Electrochemistry Redox Reactions Day 1 Section 9 1 Bleach Redox Equation a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. Write a balanced equation for the reaction of. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. h2o2 æ 1⁄2 o2 + h2o. Oxidation is most generally defined as. write a balanced equation for the reaction. Bleach Redox Equation.
From www.chegg.com
Solved SAMPLE QUESTIONS REDOX REACTIONS Balance each redox Bleach Redox Equation ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Write a balanced equation for the reaction of. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. Oxidation is most generally defined as. commercial bleaches are created by bubbling chlorine gas into a sodium. Bleach Redox Equation.
From www.numerade.com
SOLVED 1. Household bleach (NaOCl) is really a solution of sodium Bleach Redox Equation commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. Oxidation is most generally defined as. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Write a balanced equation for the. Bleach Redox Equation.
From www.chegg.com
EXPERIMENT 18 Determination of Bleach A Redox Bleach Redox Equation ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Oxidation is most generally defined as. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. write a balanced equation for the reaction of bleach with potassium iodide. Write a balanced equation for the reaction. Bleach Redox Equation.
From www.youtube.com
RedOx Writing RedOx HalfReactions YouTube Bleach Redox Equation h2o2 æ 1⁄2 o2 + h2o. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. write a balanced equation for the reaction of bleach with potassium iodide. Oxidation is most generally defined as. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. sodium hypochlorite is. Bleach Redox Equation.
From byjus.com
What is the difference between combination reaction redox and Bleach Redox Equation write a balanced equation for the reaction of bleach with potassium iodide. h2o2 æ 1⁄2 o2 + h2o. Oxidation is most generally defined as. 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. commercial bleaches are. Bleach Redox Equation.
From www.youtube.com
Redox Reaction Oxidation of Iron (II) to Iron (III) Redox Bleach Redox Equation commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. Write a balanced equation for the reaction of. h2o2 æ 1⁄2 o2 + h2o. a redox titration is conducted to determine the concentration of sodium hypochlorite in. Bleach Redox Equation.
From www.chegg.com
EXPERIMENT 18 Determination of Bleach A Redox Bleach Redox Equation 1) during this process, hydrogen peroxide oxidizes the colored compounds in the blood. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. Oxidation is most generally defined as. write a balanced equation for the reaction of bleach with potassium iodide. a redox titration is conducted to determine the concentration of. Bleach Redox Equation.
From www.chegg.com
Solved EXPERIMENT 18 Determination Of BleachA Redox Tit... Bleach Redox Equation write a balanced equation for the reaction of bleach with potassium iodide. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. Oxidation is most generally defined as. h2o2 æ 1⁄2 o2 + h2o. ordinary household bleach, sodium hypochlorite (naclo), acts on a stain through the chemical process called oxidation. a redox. Bleach Redox Equation.
From www.blendspace.com
Molecular Solids Lessons Blendspace Bleach Redox Equation sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. Oxidation is most generally defined as. a redox titration is conducted to determine the concentration of sodium hypochlorite in the bleach sample. ordinary household bleach, sodium hypochlorite. Bleach Redox Equation.
From www.aakash.ac.in
Redox Reactions Definition, Types, Applications & Uses Chemistry Bleach Redox Equation commercial bleaches are created by bubbling chlorine gas into a sodium hydroxide solution. Oxidation is most generally defined as. write a balanced equation for the reaction of bleach with potassium iodide. sodium hypochlorite is an inexpensive, strong oxidizing agent, that is used as disinfectant and bleaching agent. h2o2 æ 1⁄2 o2 + h2o. a redox. Bleach Redox Equation.