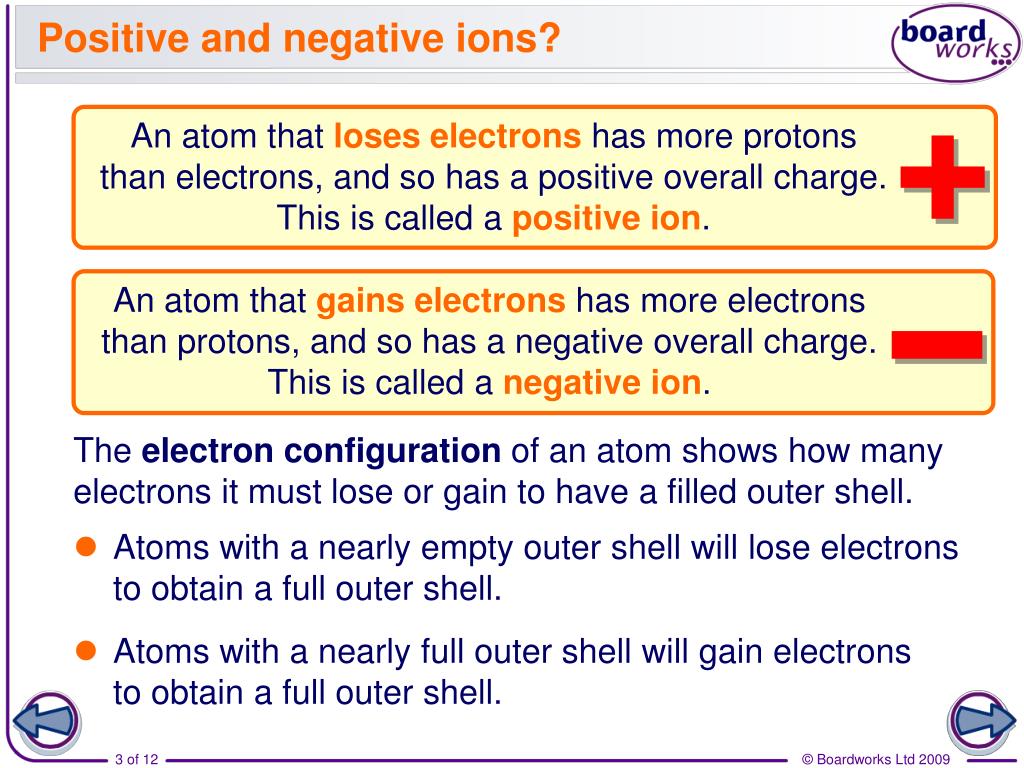
Do All Positive Ions Have More Protons Than Electrons . If the atom gains one or more electrons, it becomes a. The atom therefore now has a. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. If it has fewer electrons than protons it has a net. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. The ions formed are negative, because they have more electrons than protons. The ions have the electronic structure of a noble gas (group 0. A helium atom has two. If it has more electrons than protons it has a net negative charge and is known as an anion. The proton number is the atomic number of the element, while the electron number is the atomic number.
from www.slideserve.com
Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The atom therefore now has a. If it has more electrons than protons it has a net negative charge and is known as an anion. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. The proton number is the atomic number of the element, while the electron number is the atomic number. If it has fewer electrons than protons it has a net. The ions formed are negative, because they have more electrons than protons. The ions have the electronic structure of a noble gas (group 0. If the atom gains one or more electrons, it becomes a.
PPT How do atoms form ions? PowerPoint Presentation, free download
Do All Positive Ions Have More Protons Than Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The ions have the electronic structure of a noble gas (group 0. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. If it has more electrons than protons it has a net negative charge and is known as an anion. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The atom therefore now has a. A helium atom has two. The ions formed are negative, because they have more electrons than protons. The proton number is the atomic number of the element, while the electron number is the atomic number. If it has fewer electrons than protons it has a net. If the atom gains one or more electrons, it becomes a.
From slideplayer.com
Basic Chemistry Section 2.1 (Matter). ppt download Do All Positive Ions Have More Protons Than Electrons When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. The atom therefore now has a. The ions formed are negative, because they have more electrons than protons. If it has. Do All Positive Ions Have More Protons Than Electrons.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Do All Positive Ions Have More Protons Than Electrons The atom therefore now has a. The proton number is the atomic number of the element, while the electron number is the atomic number. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. If it has fewer electrons than protons it has a net. The ions have the electronic structure of a. Do All Positive Ions Have More Protons Than Electrons.
From courses.lumenlearning.com
Elements and Atoms The Building Blocks of Matter Anatomy and Do All Positive Ions Have More Protons Than Electrons When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The ions have the electronic structure of a noble gas (group 0. The ions formed are negative, because they have more electrons than protons. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion,. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Electricity. ppt download Do All Positive Ions Have More Protons Than Electrons If the atom gains one or more electrons, it becomes a. If it has more electrons than protons it has a net negative charge and is known as an anion. The ions formed are negative, because they have more electrons than protons. The atom therefore now has a. A helium atom has two. The loss of one or more electrons. Do All Positive Ions Have More Protons Than Electrons.
From www.worksheetsplanet.com
What is a Proton? Definition and Characteristics of Protons Do All Positive Ions Have More Protons Than Electrons If the atom gains one or more electrons, it becomes a. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The proton number is the atomic number of the element, while the electron number is the atomic number. The atom therefore now has a. When a metal atom. Do All Positive Ions Have More Protons Than Electrons.
From sciencenotes.org
What Is an Ion? Chemistry Definition Do All Positive Ions Have More Protons Than Electrons If it has more electrons than protons it has a net negative charge and is known as an anion. A helium atom has two. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. Some atoms have nearly eight electrons in their valence shell and can gain additional. Do All Positive Ions Have More Protons Than Electrons.
From www.alamy.com
Cations and Anions. Structure of ions. Examples, and Differences. Anion Do All Positive Ions Have More Protons Than Electrons The atom therefore now has a. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. If it has more electrons than protons it has a net negative charge and is known as an anion. If the atom gains one or more electrons, it becomes a. The ions have. Do All Positive Ions Have More Protons Than Electrons.
From www.nagwa.com
Question Video Comparing a Positive Ion to a Neutral Atom Nagwa Do All Positive Ions Have More Protons Than Electrons A helium atom has two. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The atom therefore now has a. The ions formed are negative, because they have more electrons than protons. If the atom gains one or more electrons, it becomes a. The ions have the electronic. Do All Positive Ions Have More Protons Than Electrons.
From www.shalom-education.com
Isotopes and Ions GCSE Physics Revision Do All Positive Ions Have More Protons Than Electrons The ions formed are negative, because they have more electrons than protons. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The proton number is the atomic number of the element,. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT Atoms and Ions PowerPoint Presentation, free download ID2050882 Do All Positive Ions Have More Protons Than Electrons Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. If it has fewer electrons than protons it has a net. The ions have the electronic structure of a noble gas (group 0. The atom therefore now has a. The proton number is the atomic number of the element,. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Elements, Atoms and Compounds ppt download Do All Positive Ions Have More Protons Than Electrons The ions formed are negative, because they have more electrons than protons. The ions have the electronic structure of a noble gas (group 0. If it has fewer electrons than protons it has a net. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. A helium atom has. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download Do All Positive Ions Have More Protons Than Electrons When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The proton number is the atomic number of the element, while the electron number is the atomic number. The ions have the electronic structure of a noble gas (group 0. The atom therefore now has a. If the atom gains one or more. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT Chapter 6 Lesson 2 Atomic Structure PowerPoint Presentation Do All Positive Ions Have More Protons Than Electrons The ions have the electronic structure of a noble gas (group 0. If it has fewer electrons than protons it has a net. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. A helium atom has two. The ions formed are negative, because they have more electrons than protons. If it has. Do All Positive Ions Have More Protons Than Electrons.
From itc.gsw.edu
Ions Do All Positive Ions Have More Protons Than Electrons The ions formed are negative, because they have more electrons than protons. If it has more electrons than protons it has a net negative charge and is known as an anion. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. If it has fewer electrons than protons it has a net. Some. Do All Positive Ions Have More Protons Than Electrons.
From www.teachoo.com
Ions Meaning and Examples [in Chemistry] Teachoo Concepts Do All Positive Ions Have More Protons Than Electrons The atom therefore now has a. The proton number is the atomic number of the element, while the electron number is the atomic number. The ions formed are negative, because they have more electrons than protons. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. Some atoms. Do All Positive Ions Have More Protons Than Electrons.
From alevelbiology.co.uk
Ions Types, Summary, Classification & Facts Do All Positive Ions Have More Protons Than Electrons If it has more electrons than protons it has a net negative charge and is known as an anion. The proton number is the atomic number of the element, while the electron number is the atomic number. The ions have the electronic structure of a noble gas (group 0. The atom therefore now has a. When a metal atom loses. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT What are bonds? PowerPoint Presentation, free download ID5861644 Do All Positive Ions Have More Protons Than Electrons If it has more electrons than protons it has a net negative charge and is known as an anion. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. If it. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Unit 3 “Atomic Structure” ppt download Do All Positive Ions Have More Protons Than Electrons The ions have the electronic structure of a noble gas (group 0. If it has fewer electrons than protons it has a net. The proton number is the atomic number of the element, while the electron number is the atomic number. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Basic Chemistry Section 2.1 (Matter). ppt download Do All Positive Ions Have More Protons Than Electrons The proton number is the atomic number of the element, while the electron number is the atomic number. If it has fewer electrons than protons it has a net. The ions have the electronic structure of a noble gas (group 0. A helium atom has two. The loss of one or more electrons results in more protons than electrons and. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT AS Chemistry Lesson 1 atomic structure PowerPoint Presentation Do All Positive Ions Have More Protons Than Electrons When a metal atom loses electrons, it ends up with a greater number of protons than electrons. A helium atom has two. The atom therefore now has a. The ions formed are negative, because they have more electrons than protons. If the atom gains one or more electrons, it becomes a. Some atoms have nearly eight electrons in their valence. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Atoms, Ions, and Isotopes ppt download Do All Positive Ions Have More Protons Than Electrons The proton number is the atomic number of the element, while the electron number is the atomic number. If it has more electrons than protons it has a net negative charge and is known as an anion. A helium atom has two. The loss of one or more electrons results in more protons than electrons and an overall positively charged. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
The Atomic Structure Chapter 1D. ppt download Do All Positive Ions Have More Protons Than Electrons The atom therefore now has a. The ions have the electronic structure of a noble gas (group 0. If it has fewer electrons than protons it has a net. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. The ions formed are negative, because they have more. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Notes Ionic Bonds 1. Key Concept Ionic bonds form when electrons are Do All Positive Ions Have More Protons Than Electrons The proton number is the atomic number of the element, while the electron number is the atomic number. The atom therefore now has a. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The loss of one or more electrons results in more protons than electrons and an. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Chapter 14 Section ppt download Do All Positive Ions Have More Protons Than Electrons If the atom gains one or more electrons, it becomes a. If it has fewer electrons than protons it has a net. A helium atom has two. The atom therefore now has a. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss of one or more electrons results in more. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Chapter 8 Chemical Bonding ppt download Do All Positive Ions Have More Protons Than Electrons The atom therefore now has a. The ions have the electronic structure of a noble gas (group 0. The proton number is the atomic number of the element, while the electron number is the atomic number. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. A helium atom. Do All Positive Ions Have More Protons Than Electrons.
From sciencenotes.org
Cations and Anions Definitions, Examples, and Differences Do All Positive Ions Have More Protons Than Electrons A helium atom has two. If the atom gains one or more electrons, it becomes a. If it has more electrons than protons it has a net negative charge and is known as an anion. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The ions formed are. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Atoms, Elements, Compounds, and Ions ppt download Do All Positive Ions Have More Protons Than Electrons If it has fewer electrons than protons it has a net. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. Some atoms have nearly eight electrons in their valence shell and can gain additional valence electrons until they have an octet. The loss of one or more electrons results in more protons. Do All Positive Ions Have More Protons Than Electrons.
From www.shalom-education.com
Atomic Structure and Ions GCSE Chemistry Revision Do All Positive Ions Have More Protons Than Electrons If it has more electrons than protons it has a net negative charge and is known as an anion. The atom therefore now has a. A helium atom has two. The proton number is the atomic number of the element, while the electron number is the atomic number. The ions formed are negative, because they have more electrons than protons.. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Notes Ionic Bonds 1. Key Concept Ionic bonds form when electrons are Do All Positive Ions Have More Protons Than Electrons The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. If it has more electrons than protons it has a net negative charge and is known as an anion. If it has fewer electrons than protons it has a net. When a metal atom loses electrons, it ends. Do All Positive Ions Have More Protons Than Electrons.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry Do All Positive Ions Have More Protons Than Electrons The ions have the electronic structure of a noble gas (group 0. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. Some atoms have nearly eight electrons in their valence. Do All Positive Ions Have More Protons Than Electrons.
From slideplayer.com
Ionic and Covalent Bonding ppt download Do All Positive Ions Have More Protons Than Electrons A helium atom has two. The ions have the electronic structure of a noble gas (group 0. The ions formed are negative, because they have more electrons than protons. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. The loss of one or more electrons results in more protons than electrons and. Do All Positive Ions Have More Protons Than Electrons.
From www.sliderbase.com
Formula of Ionic Compounds Do All Positive Ions Have More Protons Than Electrons The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. When a metal atom loses electrons, it ends up with a greater number of protons than electrons. If it has fewer electrons than protons it has a net. The ions formed are negative, because they have more electrons. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT Electron Cloud Model PowerPoint Presentation, free download ID Do All Positive Ions Have More Protons Than Electrons If it has more electrons than protons it has a net negative charge and is known as an anion. If the atom gains one or more electrons, it becomes a. The proton number is the atomic number of the element, while the electron number is the atomic number. If it has fewer electrons than protons it has a net. Some. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT Isotopes and Ions PowerPoint Presentation, free download ID6565097 Do All Positive Ions Have More Protons Than Electrons The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. The ions formed are negative, because they have more electrons than protons. If it has more electrons than protons it has a net negative charge and is known as an anion. The atom therefore now has a. Some. Do All Positive Ions Have More Protons Than Electrons.
From www.slideserve.com
PPT Atoms, Molecules & Ions PowerPoint Presentation, free download Do All Positive Ions Have More Protons Than Electrons If the atom gains one or more electrons, it becomes a. The loss of one or more electrons results in more protons than electrons and an overall positively charged ion, called a cation. If it has fewer electrons than protons it has a net. When a metal atom loses electrons, it ends up with a greater number of protons than. Do All Positive Ions Have More Protons Than Electrons.