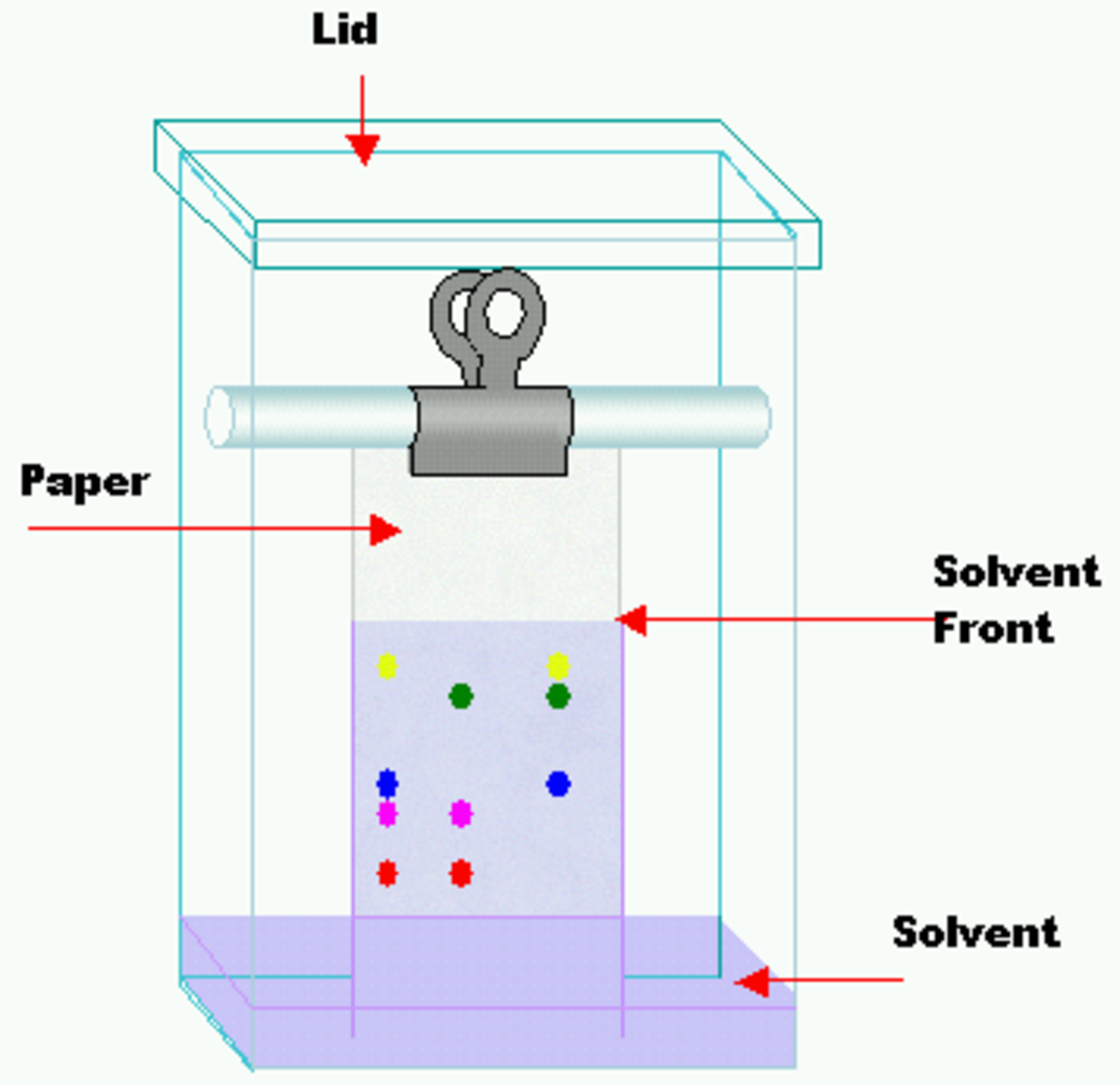
Paper Chromatography Polar Nonpolar . For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. These properties affect the solubility of the compounds and. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Paper chromatography is used for separating chemicals based on their different properties (ex: The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Commonly, chromatography paper is made of cellulose, which is a polar polymer. The solvent that is used can be either nonpolar or polar. Since the mobile phase is an. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between.
from owlcation.com
Since the mobile phase is an. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. These properties affect the solubility of the compounds and. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Commonly, chromatography paper is made of cellulose, which is a polar polymer. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Paper chromatography is used for separating chemicals based on their different properties (ex: The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The solvent that is used can be either nonpolar or polar.
What Is Paper Chromatography and How Does It Work? Owlcation
Paper Chromatography Polar Nonpolar The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Since the mobile phase is an. These properties affect the solubility of the compounds and. Commonly, chromatography paper is made of cellulose, which is a polar polymer. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The solvent that is used can be either nonpolar or polar. Paper chromatography is used for separating chemicals based on their different properties (ex: Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between.
From www.youtube.com
Paper chromatography/Radial paper chromatography (Principle, procedure Paper Chromatography Polar Nonpolar Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. The solvent that is used can be either nonpolar or polar. These properties affect the solubility of the compounds and. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). For this example, let’s say our solvent is nonpolar, and. Paper Chromatography Polar Nonpolar.
From www.shemmassianconsulting.com
Reactions and Separations for the MCAT Everything You Need to Know Paper Chromatography Polar Nonpolar The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Commonly, chromatography paper is made of cellulose, which is a polar polymer. These properties affect the solubility of the compounds and. The solvent that is used can be either. Paper Chromatography Polar Nonpolar.
From www.qeios.com
Developing A Novel Solvent System to Isolate Plant Pigments of Paper Chromatography Polar Nonpolar Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Since the mobile phase is an. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent that is used can be. Paper Chromatography Polar Nonpolar.
From acechemistry.co.uk
Chromatography Ace Chemistry Paper Chromatography Polar Nonpolar Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. The solvent that is used can be either nonpolar or polar. These properties affect the solubility of the compounds and. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent may be polar (like water), somewhat polar (like isopropyl. Paper Chromatography Polar Nonpolar.
From ar.inspiredpencil.com
Paper Chromatography Chlorophyll Paper Chromatography Polar Nonpolar Commonly, chromatography paper is made of cellulose, which is a polar polymer. These properties affect the solubility of the compounds and. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Chemicals can be very. Paper Chromatography Polar Nonpolar.
From ziarovky.com
By What Method Can Paper Chromatography Works? Zia Rovky Paper Chromatography Polar Nonpolar The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. These properties affect the solubility of the compounds and. Commonly, chromatography paper is made of cellulose, which is a polar polymer. The ability of chromatography to separate. Paper Chromatography Polar Nonpolar.
From microbenotes.com
Paper Chromatography Definition, Types, Principle, Steps, Uses Paper Chromatography Polar Nonpolar The solvent that is used can be either nonpolar or polar. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Paper chromatography is used for separating chemicals based on their different properties (ex: For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Solubility, size, mass, etc.) and thus,. Paper Chromatography Polar Nonpolar.
From www.vecteezy.com
Paper chromatography analytical method for the separation of a mixture Paper Chromatography Polar Nonpolar Since the mobile phase is an. These properties affect the solubility of the compounds and. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Commonly, chromatography paper is made of cellulose, which is a polar polymer. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Chemicals can be very. Paper Chromatography Polar Nonpolar.
From slidetodoc.com
PolarNonpolar solvents using chromatography Purpose To use paper Paper Chromatography Polar Nonpolar Since the mobile phase is an. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. These properties affect the solubility of the compounds and. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent. Paper Chromatography Polar Nonpolar.
From www.studypool.com
SOLUTION Types of paper chromatography Studypool Paper Chromatography Polar Nonpolar The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent that is used can be either nonpolar or polar. Chemicals can be very polar, very nonpolar, and all degrees of. Paper Chromatography Polar Nonpolar.
From employees.csbsju.edu
CHEM 123 Chapt 2 Paper Chromatography Polar Nonpolar The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. These properties affect the solubility of the compounds and.. Paper Chromatography Polar Nonpolar.
From mungfali.com
Paper Chromatography Labelled Diagram Paper Chromatography Polar Nonpolar Since the mobile phase is an. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Paper chromatography is used for separating chemicals based on their different properties (ex: Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. The solvent that is used can. Paper Chromatography Polar Nonpolar.
From es.slideshare.net
Chromatography (paper chromatography and tlc) Paper Chromatography Polar Nonpolar The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Paper chromatography is used for separating chemicals based on their different properties (ex: These properties affect the solubility of the compounds and. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper.. Paper Chromatography Polar Nonpolar.
From 2014howwenbin.weebly.com
Science Reflection 7 Paper Chromatography How Wen Bin Paper Chromatography Polar Nonpolar The solvent that is used can be either nonpolar or polar. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and.. Paper Chromatography Polar Nonpolar.
From sciencestuffbyamy.blogspot.com.au
Amy Brown Science Paper Chromatography Paper Chromatography Polar Nonpolar The solvent that is used can be either nonpolar or polar. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. These properties affect the solubility of the compounds and. Since the mobile phase is an. Paper chromatography is used for separating chemicals based on their different properties. Paper Chromatography Polar Nonpolar.
From www.yaclass.in
Paper chromatography — lesson. Science State Board, Class 9. Paper Chromatography Polar Nonpolar These properties affect the solubility of the compounds and. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Since the mobile phase is an. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent that is used can be either nonpolar or polar. Chemicals. Paper Chromatography Polar Nonpolar.
From www.slideserve.com
PPT Chromatography PowerPoint Presentation, free download ID542840 Paper Chromatography Polar Nonpolar These properties affect the solubility of the compounds and. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Commonly, chromatography paper is made of cellulose, which is a polar polymer. Since the mobile phase is an. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Chemicals can be very. Paper Chromatography Polar Nonpolar.
From travelintheworld.ga
Paper chromatography polarity Paper Chromatography Polar Nonpolar For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Since the mobile phase is an. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). The solvent that is used can be either nonpolar or polar. Solubility, size, mass, etc.) and thus, allows scientists to distinguish. Paper Chromatography Polar Nonpolar.
From owlcation.com
What Is Paper Chromatography Principle, Types, and Uses Owlcation Paper Chromatography Polar Nonpolar For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent that is used can be either nonpolar or polar. These properties affect the solubility of the compounds and. Paper chromatography is used for separating chemicals based on their different properties (ex: Commonly, chromatography paper is made of cellulose, which is a polar. Paper Chromatography Polar Nonpolar.
From chem.libretexts.org
2 Paper Chromatography of Gel Ink Pens (Experiment) Chemistry LibreTexts Paper Chromatography Polar Nonpolar Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Commonly, chromatography paper is made of cellulose, which is a polar polymer. Paper chromatography. Paper Chromatography Polar Nonpolar.
From owlcation.com
What Is Paper Chromatography Principle, Types, & Uses Owlcation Paper Chromatography Polar Nonpolar Commonly, chromatography paper is made of cellulose, which is a polar polymer. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. The solvent that is used can be either nonpolar or polar. These properties affect the solubility of the compounds and. Chemicals can be very polar, very. Paper Chromatography Polar Nonpolar.
From www.youtube.com
Paper Chromatography Intro & Theory YouTube Paper Chromatography Polar Nonpolar Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Commonly, chromatography paper is made of cellulose, which is a polar polymer. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Paper chromatography is used. Paper Chromatography Polar Nonpolar.
From wordwall.net
Paper chromatography Labelled diagram Paper Chromatography Polar Nonpolar Commonly, chromatography paper is made of cellulose, which is a polar polymer. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent that is used can be either nonpolar or polar. Paper chromatography is used for separating chemicals based on their different properties (ex: Since the mobile phase is an. These properties. Paper Chromatography Polar Nonpolar.
From owlcation.com
What Is Paper Chromatography Principle, Types, and Uses Owlcation Paper Chromatography Polar Nonpolar Paper chromatography is used for separating chemicals based on their different properties (ex: Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Since the mobile phase is an. These properties affect the solubility of the compounds and. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Chemicals can be. Paper Chromatography Polar Nonpolar.
From chemfionaflora.blogspot.com
Chem Is Fun Lab Separation of a Mixture by Paper Chromatography Paper Chromatography Polar Nonpolar Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. These properties affect the solubility of the compounds and. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. For this. Paper Chromatography Polar Nonpolar.
From slidetodoc.com
Colors in Chemistry Learning Polarity with Paper Chromatography Paper Chromatography Polar Nonpolar For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. These properties affect the solubility of the compounds and. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil). Commonly, chromatography paper is made. Paper Chromatography Polar Nonpolar.
From www.sciencephoto.com
Paper chromatography Stock Image A500/0258 Science Photo Library Paper Chromatography Polar Nonpolar Paper chromatography is used for separating chemicals based on their different properties (ex: For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. These properties affect the solubility of the compounds and. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Chemicals can be very polar, very nonpolar, and all. Paper Chromatography Polar Nonpolar.
From www.youtube.com
Molecular Polarity used in Chromatography YouTube Paper Chromatography Polar Nonpolar Since the mobile phase is an. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Commonly, chromatography paper is made of cellulose, which is a polar polymer. Paper chromatography is used for separating chemicals based on their different properties (ex: The solvent. Paper Chromatography Polar Nonpolar.
From narodnatribuna.info
Paper Chromatography Definition Principles Procedure And Paper Chromatography Polar Nonpolar For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like vegetable oil).. Paper Chromatography Polar Nonpolar.
From biochemden.com
What is Paper Chromatography? Principle and Procedure Paper Chromatography Polar Nonpolar Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. These properties affect the solubility of the compounds and. Since the mobile phase is an. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. Paper chromatography is used for separating chemicals based on their different properties (ex: The solvent that is used. Paper Chromatography Polar Nonpolar.
From owlcation.com
What Is Paper Chromatography and How Does It Work? Owlcation Paper Chromatography Polar Nonpolar These properties affect the solubility of the compounds and. Paper chromatography is used for separating chemicals based on their different properties (ex: Since the mobile phase is an. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Chemicals can be very polar, very nonpolar, and all degrees. Paper Chromatography Polar Nonpolar.
From www.slideserve.com
PPT Gas Chromatography PowerPoint Presentation, free download ID298459 Paper Chromatography Polar Nonpolar The solvent that is used can be either nonpolar or polar. Solubility, size, mass, etc.) and thus, allows scientists to distinguish various organic and. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Since the mobile phase is an. The solvent may be polar (like water), somewhat. Paper Chromatography Polar Nonpolar.
From slidetodoc.com
Colors in Chemistry Learning Polarity with Paper Chromatography Paper Chromatography Polar Nonpolar Commonly, chromatography paper is made of cellulose, which is a polar polymer. The ability of chromatography to separate components in a mixture depends on equilibration of a compound between the stationary and mobile phases. Chemicals can be very polar, very nonpolar, and all degrees of polar/nonpolar in between. Paper chromatography is used for separating chemicals based on their different properties. Paper Chromatography Polar Nonpolar.
From www.youtube.com
Polarity Demonstration Paper Chromatography Video 2 YouTube Paper Chromatography Polar Nonpolar Since the mobile phase is an. Commonly, chromatography paper is made of cellulose, which is a polar polymer. The solvent that is used can be either nonpolar or polar. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent may be polar (like water), somewhat polar (like isopropyl alcohol), or nonpolar (like. Paper Chromatography Polar Nonpolar.
From biochemden.com
What is Paper Chromatography? Principle and Procedure Paper Chromatography Polar Nonpolar Commonly, chromatography paper is made of cellulose, which is a polar polymer. For this example, let’s say our solvent is nonpolar, and we are using polar chromatography paper. The solvent that is used can be either nonpolar or polar. Paper chromatography is used for separating chemicals based on their different properties (ex: Solubility, size, mass, etc.) and thus, allows scientists. Paper Chromatography Polar Nonpolar.