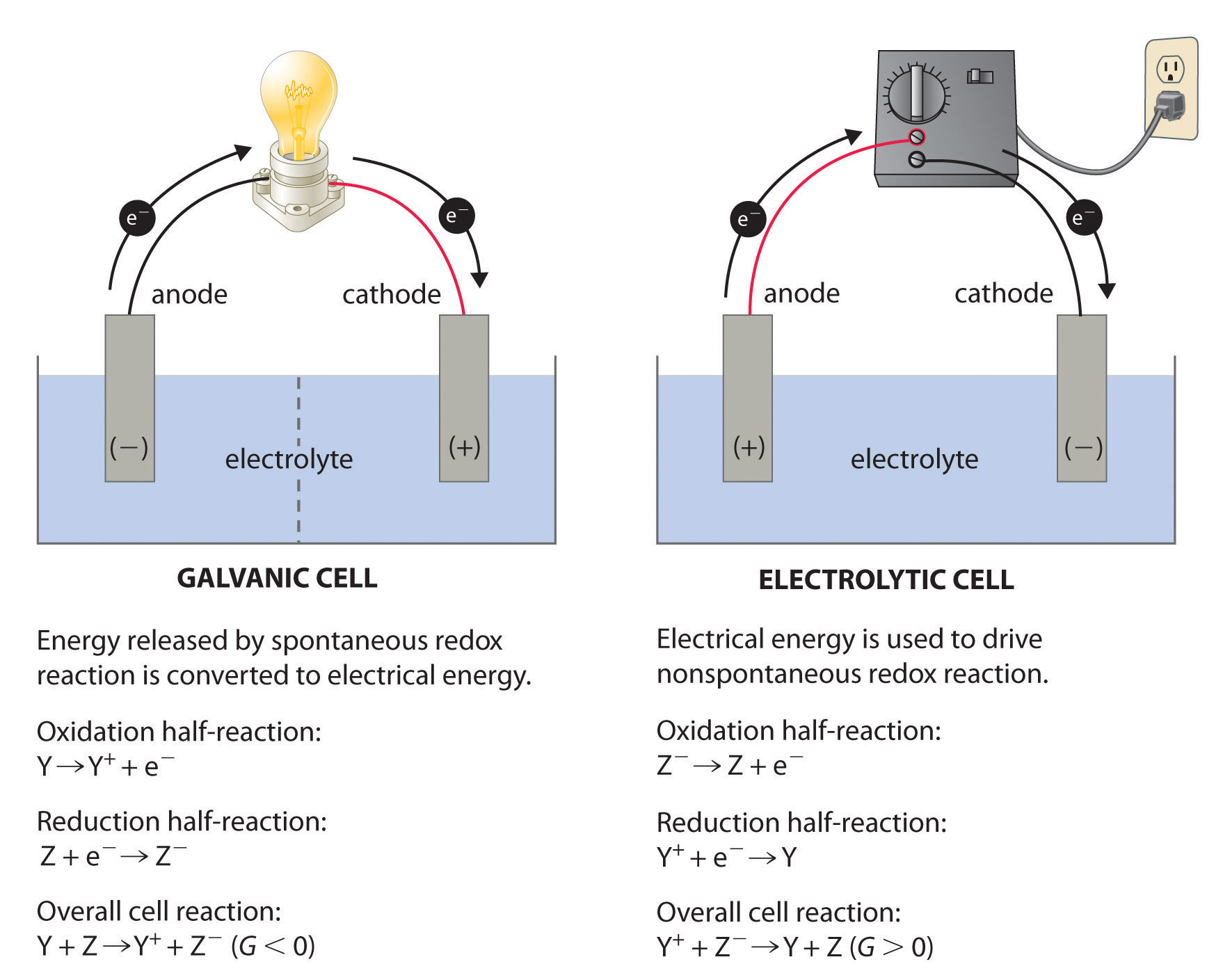
Hydrogen Fuel Cell Electrochemistry . This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Hydrogen is oxidized to protons at the anode, and the electrons are. Oxygen is supplied to a similar electrode except that the catalyst is silver. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons.
from chem.libretexts.org
A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen is oxidized to protons at the anode, and the electrons are. Oxygen is supplied to a similar electrode except that the catalyst is silver.
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts
Hydrogen Fuel Cell Electrochemistry A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. Oxygen is supplied to a similar electrode except that the catalyst is silver. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen is oxidized to protons at the anode, and the electrons are. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. A hydrogen fuel cell produces electrical energy directly from a chemical reaction.
From www.youtube.com
Hydrogen Oxygen Fuel Cell Electrochemistry YouTube Hydrogen Fuel Cell Electrochemistry In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen is oxidized to protons at the anode, and the electrons are. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode. Hydrogen Fuel Cell Electrochemistry.
From www.dreamstime.com
Hydrogen Fuel Cell Electrode Reactions Stock Photo Image of Hydrogen Fuel Cell Electrochemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the. Hydrogen Fuel Cell Electrochemistry.
From www.britannica.com
fuel cell summary Britannica Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. In the case of fuel. Hydrogen Fuel Cell Electrochemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Hydrogen Fuel Cell Electrochemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen is oxidized to protons at the anode, and the. Hydrogen Fuel Cell Electrochemistry.
From totalshield.com
Electrolyzer and Hydrogen Fuel Cell Safety TotalShield Hydrogen Fuel Cell Electrochemistry In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen. Hydrogen Fuel Cell Electrochemistry.
From chemistry.stackexchange.com
electrochemistry Hydrogen fuel cell why do the H+ ions move through Hydrogen Fuel Cell Electrochemistry A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen is oxidized to. Hydrogen Fuel Cell Electrochemistry.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Hydrogen Fuel Cell Electrochemistry In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is silver. Hydrogen is oxidized to protons at the anode, and the electrons. Hydrogen Fuel Cell Electrochemistry.
From www.sundyne.com
Hydrogen Fuel Cells How Do They Work? Sundyne Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Oxygen is supplied to a similar electrode except that the catalyst is silver. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen is oxidized to protons at the anode, and the electrons are. In the case of fuel cells. Hydrogen Fuel Cell Electrochemistry.
From www.lapsedphysicist.org
Hydrogen and Fuel Cells Important Parts of Our Energy Future Hydrogen Fuel Cell Electrochemistry A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen is oxidized to protons at the anode, and the electrons are. In the case of fuel cells (left side), hydrogen fuel. Hydrogen Fuel Cell Electrochemistry.
From www.youtube.com
Hydrogen Fuel Cell Electrochemistry 5070/0620 YouTube Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Oxygen is supplied to a similar electrode except. Hydrogen Fuel Cell Electrochemistry.
From www.youtube.com
Hydrogen & Fuel Cells Reactions Chemistry FuseSchool YouTube Hydrogen Fuel Cell Electrochemistry In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Oxygen is supplied to a similar electrode except that the catalyst is silver. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the. Hydrogen Fuel Cell Electrochemistry.
From www.pinterest.com
Chapter 3 Electrochemistry build a hydrogen fuel cell Hydrogen Hydrogen Fuel Cell Electrochemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen is oxidized to protons at the anode, and the electrons are.. Hydrogen Fuel Cell Electrochemistry.
From mmerevise.co.uk
Commercial Applications of Electrochemical Cells MME Hydrogen Fuel Cell Electrochemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is silver. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. This chapter is devoted to recapping the fundamental electrochemistry for fuel. Hydrogen Fuel Cell Electrochemistry.
From www.alamy.com
Fuel cell diagram. Vector. Device that converts chemical potential Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Oxygen is supplied to a similar electrode except that the catalyst is silver. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing. Hydrogen Fuel Cell Electrochemistry.
From www.peakscientific.com
How does a Hydrogen generator work? Hydrogen Fuel Cell Electrochemistry A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid. Hydrogen Fuel Cell Electrochemistry.
From saylordotorg.github.io
Electrochemistry Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Hydrogen is oxidized to protons at the anode, and the electrons are. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Oxygen is supplied to a similar electrode except that the. Hydrogen Fuel Cell Electrochemistry.
From climatebiz.com
How To Build A DIY Hydrogen Fuel Cell Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. A hydrogen fuel cell produces electrical energy. Hydrogen Fuel Cell Electrochemistry.
From www.studypool.com
SOLUTION Hydrogen Fuel Cells IGCSE Chemistry Studypool Hydrogen Fuel Cell Electrochemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell is. Hydrogen Fuel Cell Electrochemistry.
From www.narayannepal.com.np
Electrochemistry Class 12 Chemistry Notes NEB Best NEB Notes Hydrogen Fuel Cell Electrochemistry Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen enters the cell. Hydrogen Fuel Cell Electrochemistry.
From saylordotorg.github.io
Electrochemistry Hydrogen Fuel Cell Electrochemistry A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Oxygen is supplied to a similar electrode except that the catalyst is silver. Hydrogen is oxidized to protons at the anode, and the electrons are. In the case of fuel cells (left side),. Hydrogen Fuel Cell Electrochemistry.
From www.pinterest.com
Image result for explain about fuel cells Electrochemistry, Fuel cell Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Oxygen is supplied to a similar electrode except that the catalyst is silver. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell. Hydrogen Fuel Cell Electrochemistry.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Hydrogen Fuel Cell Electrochemistry This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen is oxidized to protons at the. Hydrogen Fuel Cell Electrochemistry.
From www.researchgate.net
e Schematic view of a typical hydrogen PEM fuel cell and its components Hydrogen Fuel Cell Electrochemistry Oxygen is supplied to a similar electrode except that the catalyst is silver. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing. Hydrogen Fuel Cell Electrochemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Hydrogen Fuel Cell Electrochemistry In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. Oxygen is supplied to. Hydrogen Fuel Cell Electrochemistry.
From large.stanford.edu
Hydrogen Fuel Cells Hydrogen Fuel Cell Electrochemistry A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells. Hydrogen Fuel Cell Electrochemistry.
From scitoys.com
Chapter 3 Electrochemistry build a hydrogen fuel cell Hydrogen Fuel Cell Electrochemistry In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Oxygen is supplied to a similar electrode except that the catalyst is silver. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. Hydrogen enters the. Hydrogen Fuel Cell Electrochemistry.
From www.scribd.com
Electrochemical Hydrogen Pumping Book PDF Fuel Cell Electrochemistry Hydrogen Fuel Cell Electrochemistry A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Oxygen is supplied to a similar electrode except that the catalyst is silver. Hydrogen enters the cell through a porous carbon electrode which also contains. Hydrogen Fuel Cell Electrochemistry.
From scitoys.com
Chapter 3 Electrochemistry build a hydrogen fuel cell Hydrogen Fuel Cell Electrochemistry Oxygen is supplied to a similar electrode except that the catalyst is silver. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Hydrogen is oxidized. Hydrogen Fuel Cell Electrochemistry.
From revisechemistry.uk
Chemical Cells and Fuel Cells AQA C5 revisechemistry.uk Hydrogen Fuel Cell Electrochemistry Hydrogen is oxidized to protons at the anode, and the electrons are. Oxygen is supplied to a similar electrode except that the catalyst is silver. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum. Hydrogen Fuel Cell Electrochemistry.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) Hydrogen Fuel Cell Electrochemistry In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. Oxygen. Hydrogen Fuel Cell Electrochemistry.
From www.electroniclinic.com
Hydrogen Fuel Cell, Application of Fuel Cells, construction, and Working Hydrogen Fuel Cell Electrochemistry Oxygen is supplied to a similar electrode except that the catalyst is silver. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a. Hydrogen Fuel Cell Electrochemistry.
From www.youtube.com
Electrochemistry 14 Fuel Cell Hydrogen Oxygen fuel Cell YouTube Hydrogen Fuel Cell Electrochemistry Hydrogen is oxidized to protons at the anode, and the electrons are. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode potential and. Oxygen is supplied to a similar electrode except that the catalyst is silver. A fuel cell is an electrochemical. Hydrogen Fuel Cell Electrochemistry.
From www.vedantu.com
What are ‘fuel cells’? Write cathode and anode reaction in a fuel cell. Hydrogen Fuel Cell Electrochemistry Hydrogen enters the cell through a porous carbon electrode which also contains a platinum catalyst. Hydrogen is oxidized to protons at the anode, and the electrons are. A hydrogen fuel cell produces electrical energy directly from a chemical reaction. Oxygen is supplied to a similar electrode except that the catalyst is silver. A fuel cell is an electrochemical device, converting. Hydrogen Fuel Cell Electrochemistry.
From stock.adobe.com
Block diagram of fuel cell. Schematic diagram of hydrogen fuel cell Hydrogen Fuel Cell Electrochemistry Oxygen is supplied to a similar electrode except that the catalyst is silver. Hydrogen is oxidized to protons at the anode, and the electrons are. A fuel cell is an electrochemical device, converting the chemical energy (gibbs free energy ) stored in a gaseous or liquid fuel, e.g.,. Hydrogen enters the cell through a porous carbon electrode which also contains. Hydrogen Fuel Cell Electrochemistry.
From www.youtube.com
EC24/TN12th STD/Fuel Cells H2O2 Functioning of Fuel cells/Secondary Hydrogen Fuel Cell Electrochemistry A hydrogen fuel cell produces electrical energy directly from a chemical reaction. In the case of fuel cells (left side), hydrogen fuel is supplied and oxidized at the anode, producing protons and electrons. Hydrogen is oxidized to protons at the anode, and the electrons are. This chapter is devoted to recapping the fundamental electrochemistry for fuel cells including the electrode. Hydrogen Fuel Cell Electrochemistry.