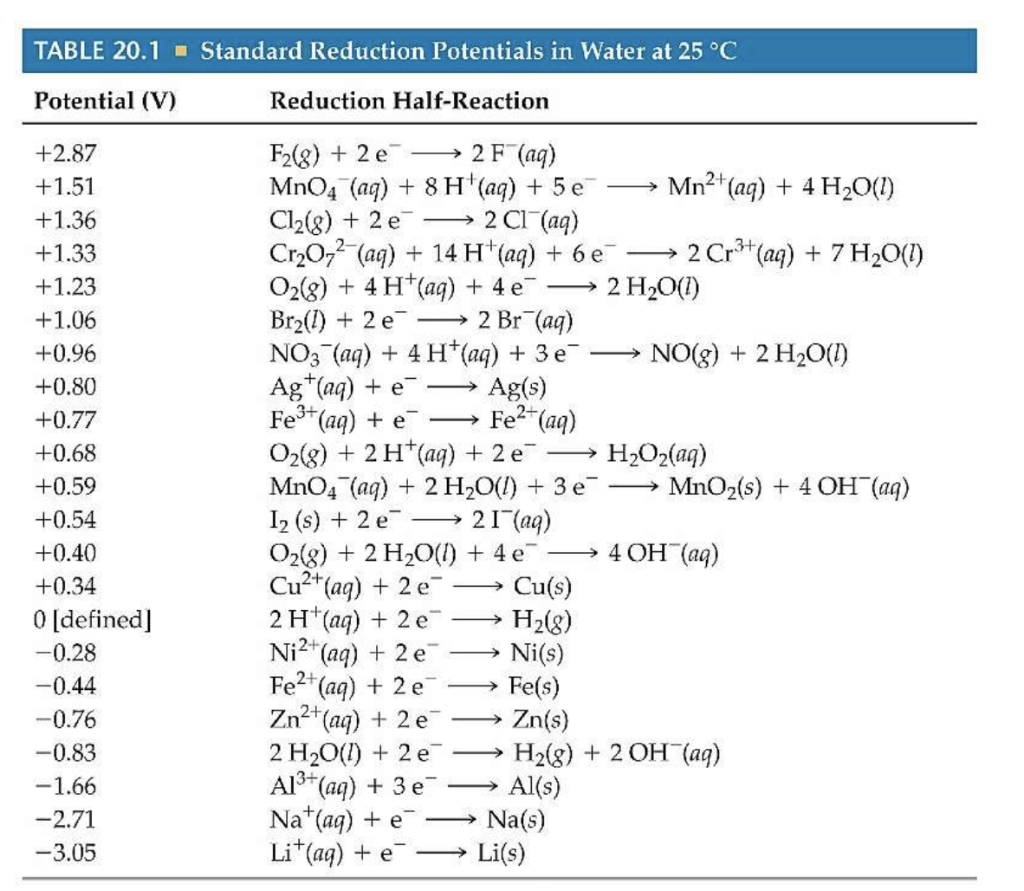
Standard Electrode Potential Cell Reaction . It has a cell potential of 0.00v, measured under standard. Important standard electrode (reduction) potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). Describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. The table below is a list of important standard electrode potentials in the reduction state. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; Describe and relate the definitions of electrode and cell potentials. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. When using the half cells below, instead of.
from www.tpsearchtool.com
The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. It has a cell potential of 0.00v, measured under standard. When using the half cells below, instead of. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage.
Standard Reduction Potential Charts For Chemistry Images
Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. When using the half cells below, instead of. Describe and relate the definitions of electrode and cell potentials. Describe and relate the definitions of electrode and cell potentials. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The table below is a list of important standard electrode potentials in the reduction state. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. Important standard electrode (reduction) potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). It has a cell potential of 0.00v, measured under standard.
From app.pandai.org
Standard Electrode Potential Standard Electrode Potential Cell Reaction The table below is a list of important standard electrode potentials in the reduction state. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. To determine oxidation electrodes, the reduction. Standard Electrode Potential Cell Reaction.
From app.pandai.org
Determine oxidising agent and reducing agent based on value of standard Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. When using the half cells below, instead of. Describe and relate the definitions of electrode and cell potentials. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The table below is a list of important standard electrode potentials in the reduction state.. Standard Electrode Potential Cell Reaction.
From www.numerade.com
SOLVED Use tabulated standard electrode potentials to calculate the Standard Electrode Potential Cell Reaction The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in. Standard Electrode Potential Cell Reaction.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint Standard Electrode Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. The table below is a list of important standard electrode potentials in the reduction state. It has a cell potential of 0.00v, measured under standard. Consequently, it is a measure of the driving force for the reaction, which earlier. Standard Electrode Potential Cell Reaction.
From www.vrogue.co
Electrochemistry Electrode Potential And Standard Ele vrogue.co Standard Electrode Potential Cell Reaction The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. Important standard electrode (reduction) potentials. Describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to. Standard Electrode Potential Cell Reaction.
From www.careerpower.in
Electrode Potential Definition, Formula, Standard Electrode Potential Standard Electrode Potential Cell Reaction The table below is a list of important standard electrode potentials in the reduction state. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. Important standard electrode (reduction) potentials. When using the half cells below, instead of. It has a cell potential of 0.00v, measured under standard. Consequently,. Standard Electrode Potential Cell Reaction.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 Standard Electrode Potential Cell Reaction The table below is a list of important standard electrode potentials in the reduction state. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. When using the half cells below, instead of. Interpret electrode potentials in terms of relative oxidant and reductant strengths. To determine oxidation electrodes,. Standard Electrode Potential Cell Reaction.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Cell Reaction The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. Describe and relate the definitions of electrode and cell potentials. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. It has a cell potential of 0.00v,. Standard Electrode Potential Cell Reaction.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Electrode Potential Cell Reaction Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. Standard Electrode Potential Cell Reaction.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; Interpret electrode potentials in. Standard Electrode Potential Cell Reaction.
From www.toppr.com
For the cell reaction 2Cu^ + (aq)→ Cu(s) + Cu^2 + (aq) , the standard Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). Describe and relate the definitions of electrode and cell potentials. Important standard electrode (reduction) potentials. The table below is a list of important standard electrode potentials in the. Standard Electrode Potential Cell Reaction.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. The table below is a list of important standard electrode potentials in the reduction state. The standard cell potential for a redox reaction (e° cell) is a measure. Standard Electrode Potential Cell Reaction.
From 2012books.lardbucket.org
Standard Potentials Standard Electrode Potential Cell Reaction When using the half cells below, instead of. Describe and relate the definitions of electrode and cell potentials. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. Standard Electrode Potential Cell Reaction.
From pandai.me
Standard Electrode Potential Standard Electrode Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. Important standard electrode (reduction) potentials. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. To determine oxidation electrodes, the. Standard Electrode Potential Cell Reaction.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry for Majors Standard Electrode Potential Cell Reaction The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in. Standard Electrode Potential Cell Reaction.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Standard Electrode Potential Cell Reaction The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; Interpret electrode potentials in terms of relative oxidant and reductant strengths. Interpret electrode potentials in terms of relative oxidant and reductant. To determine oxidation electrodes, the reduction equation can simply be. Standard Electrode Potential Cell Reaction.
From www.slideserve.com
PPT Electrochemical Cells Voltage (Electric potential) PowerPoint Standard Electrode Potential Cell Reaction Important standard electrode (reduction) potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their. Standard Electrode Potential Cell Reaction.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Electrode Potential Cell Reaction The table below is a list of important standard electrode potentials in the reduction state. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. When using the half cells below, instead of. Important standard electrode (reduction) potentials. Describe and relate the definitions of electrode and cell potentials.. Standard Electrode Potential Cell Reaction.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Electrode Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant strengths. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). The table below is a list of important standard electrode potentials in the reduction state. Describe and relate the definitions of electrode and cell potentials. Consequently, it. Standard Electrode Potential Cell Reaction.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Electrode Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant. Interpret electrode potentials in terms of relative oxidant and reductant strengths. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). When using the half cells below, instead of. Important standard electrode (reduction) potentials. Describe and relate the. Standard Electrode Potential Cell Reaction.
From notes.pegas.is
Types of Reactions Pega's Notes Standard Electrode Potential Cell Reaction It has a cell potential of 0.00v, measured under standard. Interpret electrode potentials in terms of relative oxidant and reductant. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants. Standard Electrode Potential Cell Reaction.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Electrode Potential Cell Reaction Important standard electrode (reduction) potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). When using the half cells below,. Standard Electrode Potential Cell Reaction.
From meteonmendez.blogspot.com
Standard Electrode Potential Table MeteonMendez Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. Important standard electrode (reduction) potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The table below is a list of important standard electrode potentials in the reduction state. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their. Standard Electrode Potential Cell Reaction.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Electrode Potential Cell Reaction Important standard electrode (reduction) potentials. It has a cell potential of 0.00v, measured under standard. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard. Standard Electrode Potential Cell Reaction.
From askfilo.com
The standard electrode potentials, E∘ for the half cell reactions are as Standard Electrode Potential Cell Reaction Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. Important standard electrode (reduction) potentials. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). The standard cell potential for a redox reaction (e° cell) is a measure of the tendency. Standard Electrode Potential Cell Reaction.
From www.youtube.com
19.1 Calculate cell potentials using standard electrode potentials [HL Standard Electrode Potential Cell Reaction To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). It has a cell potential of 0.00v, measured under standard. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. Describe and relate the definitions of. Standard Electrode Potential Cell Reaction.
From dxofmrhhh.blob.core.windows.net
Standard Electrode Potential Pdf at Arthur Baker blog Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; It has a cell potential of 0.00v, measured under standard. When using the half cells below, instead of. The table below. Standard Electrode Potential Cell Reaction.
From users.highland.edu
Standard Potentials Standard Electrode Potential Cell Reaction The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; It has a cell potential of 0.00v, measured under standard. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice. Standard Electrode Potential Cell Reaction.
From www.numerade.com
SOLVED 2. Use the standard electrode potentials to calculate the cell Standard Electrode Potential Cell Reaction It has a cell potential of 0.00v, measured under standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Important standard electrode (reduction) potentials. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states. The standard cell potential for a redox reaction (e° cell) is a. Standard Electrode Potential Cell Reaction.
From youtube.com
19.1.4 Predict whether a reaction will be spontaneous using standard Standard Electrode Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant strengths. When using the half cells below, instead of. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard states to form products in their standard states; Consequently, it is a measure of the driving force for the reaction,. Standard Electrode Potential Cell Reaction.
From www.substech.com
standard_electrode_potential.png [SubsTech] Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. The table below is a list of important standard electrode potentials in the reduction state. Describe and relate the definitions of electrode and cell potentials. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. To determine oxidation electrodes, the reduction equation can. Standard Electrode Potential Cell Reaction.
From www.pveducation.org
Standard Potential PVEducation Standard Electrode Potential Cell Reaction The table below is a list of important standard electrode potentials in the reduction state. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive. Standard Electrode Potential Cell Reaction.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Electrode Potential Cell Reaction Interpret electrode potentials in terms of relative oxidant and reductant strengths. It has a cell potential of 0.00v, measured under standard. Important standard electrode (reduction) potentials. The table below is a list of important standard electrode potentials in the reduction state. Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The standard. Standard Electrode Potential Cell Reaction.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Electrode Potential Cell Reaction Describe and relate the definitions of electrode and cell potentials. To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The standard cell potential for a redox reaction (e° cell) is. Standard Electrode Potential Cell Reaction.
From edurev.in
Electrode Potentials Chemistry for Grade 12 PDF Download Standard Electrode Potential Cell Reaction To determine oxidation electrodes, the reduction equation can simply be flipped and its potential changed from positive to negative (and vice versa). Consequently, it is a measure of the driving force for the reaction, which earlier we called voltage. The standard cell potential for a redox reaction (e° cell) is a measure of the tendency of reactants in their standard. Standard Electrode Potential Cell Reaction.