Fda Definition Medical Device Manufacturer . — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — department of health and human services. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Digital health center of excellence. approvals and clearances, information on medical devices by type.
from www.greenlight.guru
— the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Digital health center of excellence. approvals and clearances, information on medical devices by type. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — department of health and human services.
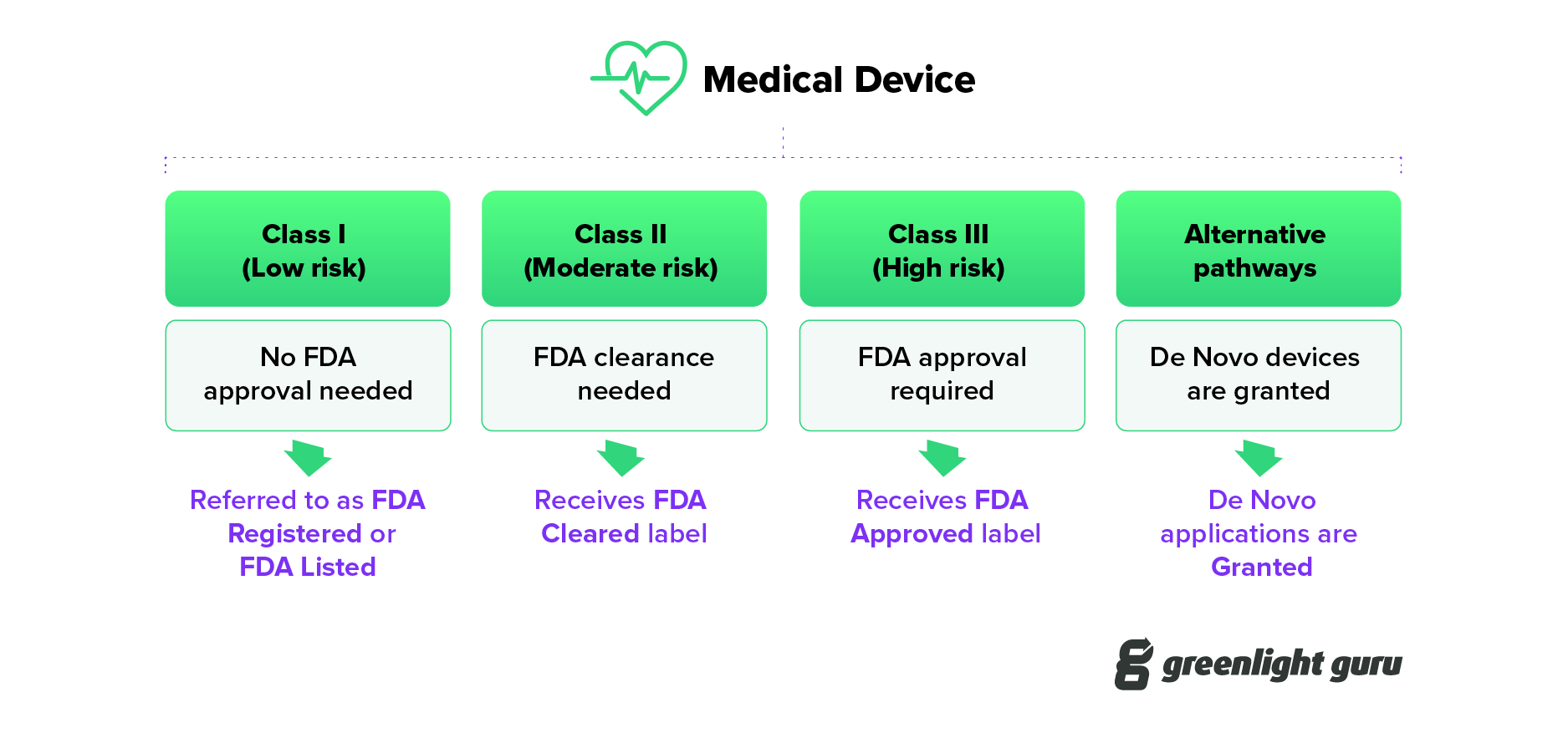
FDA Cleared vs Approved vs Granted for Medical Devices
Fda Definition Medical Device Manufacturer — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. approvals and clearances, information on medical devices by type. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. — department of health and human services. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. Digital health center of excellence. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Definition Medical Device Manufacturer (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — department of health and human services. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. Digital health center of excellence. — the fda's definition of a medical device is based on. Fda Definition Medical Device Manufacturer.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Fda Definition Medical Device Manufacturer Digital health center of excellence. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes. Fda Definition Medical Device Manufacturer.
From dxopgsnjb.blob.core.windows.net
Medical Device Manufacturer Fda at William Schafer blog Fda Definition Medical Device Manufacturer (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — medical devices are classified both by. Fda Definition Medical Device Manufacturer.
From www.medtechimpact.com
FDA Focuses on Medical Device Safety • Medtech Impact On Wellness Fda Definition Medical Device Manufacturer (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — department of health and human services. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. Digital health center of excellence. — medical devices are classified both by medical specialty. Fda Definition Medical Device Manufacturer.
From dxopgsnjb.blob.core.windows.net
Medical Device Manufacturer Fda at William Schafer blog Fda Definition Medical Device Manufacturer — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. — department of health and human services. — the fda's definition of a medical device is based on the definition found in section. Fda Definition Medical Device Manufacturer.
From barcode-labels.com
Medical Devices Electronic Imaging Materials Fda Definition Medical Device Manufacturer Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. Digital health center of excellence. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or. Fda Definition Medical Device Manufacturer.
From dxobojlav.blob.core.windows.net
Medical Device Design Requirements at Esmeralda Jones blog Fda Definition Medical Device Manufacturer — department of health and human services. Digital health center of excellence. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. Determine if your product meets the definition of a medical. Fda Definition Medical Device Manufacturer.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Fda Definition Medical Device Manufacturer — department of health and human services. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. Digital health center of excellence. (o) manufacturer means any person who designs, manufactures, fabricates,. Fda Definition Medical Device Manufacturer.
From exoxfgctr.blob.core.windows.net
India Medical Equipment Manufacturers at Aida Valazquez blog Fda Definition Medical Device Manufacturer approvals and clearances, information on medical devices by type. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes. Fda Definition Medical Device Manufacturer.
From dxoebjapk.blob.core.windows.net
What Does Device Manufacturer Mean at James Pierce blog Fda Definition Medical Device Manufacturer — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Digital health center of excellence. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — department of health and human services. — medical devices are classified both by medical specialty. Fda Definition Medical Device Manufacturer.
From protechdesign101.blogspot.com
PROTECH Design & Manufacturing, Inc. What is the Definition of Fda Definition Medical Device Manufacturer — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Digital health center. Fda Definition Medical Device Manufacturer.
From vanessapaterson.pages.dev
Medical Device Regulatory Conferences 2025 Vanessa Paterson Fda Definition Medical Device Manufacturer Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. approvals and clearances, information on medical devices by type. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or. Fda Definition Medical Device Manufacturer.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Fda Definition Medical Device Manufacturer approvals and clearances, information on medical devices by type. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — (o) manufacturer means any person who. Fda Definition Medical Device Manufacturer.
From medtechintelligence.com
FDA To Host Town Hall on Medical Device Sterilization MedTech Fda Definition Medical Device Manufacturer — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. Digital health center of excellence. approvals and clearances, information on medical devices by type. — department. Fda Definition Medical Device Manufacturer.
From vem-medical.com
Medical Device Manufacturing Fda Definition Medical Device Manufacturer — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. Determine if your product meets the definition of a medical device per section 201 (h) of the food,. Fda Definition Medical Device Manufacturer.
From www.investopedia.com
Food and Drug Administration (FDA) What It Is and Does Fda Definition Medical Device Manufacturer — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. approvals and clearances, information on medical devices by type. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — department of health and human services. — (o) manufacturer means. Fda Definition Medical Device Manufacturer.
From cmsmedtech.com
Medical Device Registration in Thailand CMS MedTech Fda Definition Medical Device Manufacturer Digital health center of excellence. approvals and clearances, information on medical devices by type. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — (o) manufacturer means any person who. Fda Definition Medical Device Manufacturer.
From blog.minitab.com
Understanding the 5 FDA Stages for Medical Device Manufacturing The Fda Definition Medical Device Manufacturer — department of health and human services. Digital health center of excellence. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. approvals and clearances, information on medical devices by type. — medical devices are classified both. Fda Definition Medical Device Manufacturer.
From www.presentationeze.com
FDA Regulatory Requirements Medical Devices.PresentationEZE Fda Definition Medical Device Manufacturer — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Digital health center. Fda Definition Medical Device Manufacturer.
From www.greenlight.guru
Am I Complying with FDA Medical Device Labeling Requirements? Fda Definition Medical Device Manufacturer Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. . Fda Definition Medical Device Manufacturer.
From www.youtube.com
FDA Regulation of Medical Devices and Software/Apps YouTube Fda Definition Medical Device Manufacturer Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. . Fda Definition Medical Device Manufacturer.
From old.sermitsiaq.ag
Medical Device Label Template Fda Definition Medical Device Manufacturer (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. approvals and clearances, information on medical devices by type. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or. Fda Definition Medical Device Manufacturer.
From www.proximacro.com
510(k) or PMA Should Your Medical Device Receive FDA Clearance or FDA Fda Definition Medical Device Manufacturer — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. Digital health center of excellence. — department of health and human services. — (o) manufacturer means any person who designs, manufactures,. Fda Definition Medical Device Manufacturer.
From www.druganddevicelawblog.com
New FDA Draft Guidance Helps Define the Scope of §510(k) Medical Device Fda Definition Medical Device Manufacturer Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. approvals and clearances, information on medical devices by type. — medical devices are classified both by medical specialty (i.e., classification panels). Fda Definition Medical Device Manufacturer.
From www.slideshare.net
Understanding FDA Requirements Medical Devices Fda Definition Medical Device Manufacturer — department of health and human services. approvals and clearances, information on medical devices by type. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. (o) manufacturer means any. Fda Definition Medical Device Manufacturer.
From knconsultingandservices.com
What is Labelling? Medical Device Consulting Company Fda Definition Medical Device Manufacturer — department of health and human services. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — (o) manufacturer means any person who designs, manufactures,. Fda Definition Medical Device Manufacturer.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Fda Definition Medical Device Manufacturer approvals and clearances, information on medical devices by type. — department of health and human services. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — the fda's definition of a medical device is based on. Fda Definition Medical Device Manufacturer.
From exovighly.blob.core.windows.net
Fda Medical Device Private Label Distributor at Jerry Robertson blog Fda Definition Medical Device Manufacturer Digital health center of excellence. approvals and clearances, information on medical devices by type. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. — department of health and human services. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. —. Fda Definition Medical Device Manufacturer.
From www.youtube.com
FDA QSR Compliance for Medical Device Manufacturers FDA Consultant Fda Definition Medical Device Manufacturer approvals and clearances, information on medical devices by type. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Digital health center of excellence. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. — medical devices are classified both by. Fda Definition Medical Device Manufacturer.
From www.kolabtree.com
A guide to FDA Design Controls for your medical device Fda Definition Medical Device Manufacturer approvals and clearances, information on medical devices by type. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — department of health and human services. Digital health center of excellence.. Fda Definition Medical Device Manufacturer.
From www.coolblue.com
What’s the Difference? FDA Form 483 Observations and Warning Letters Fda Definition Medical Device Manufacturer Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. Digital health center of excellence. approvals and clearances, information on medical devices by type. . Fda Definition Medical Device Manufacturer.
From www.regdesk.co
HSA Guidance on Grouping of Medical Devices Overview RegDesk Fda Definition Medical Device Manufacturer — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — the fda's definition of a medical device is based on the definition found in section 201(h) of the food, drug,. Determine if your product meets the definition of a medical device per section 201 (h) of the food,. Fda Definition Medical Device Manufacturer.
From blog.sierralabs.com
ISO 13485 Regulatory Requirements on Medical Devices Fda Definition Medical Device Manufacturer Digital health center of excellence. (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a finished. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — the fda's definition of a medical device is based on the definition found in section 201(h). Fda Definition Medical Device Manufacturer.
From www.mokomedtech.com
How to Get FDA Approval for Medical Devices MokoMedtech Professional Fda Definition Medical Device Manufacturer — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. approvals and clearances, information on medical devices by type. — department of health and human services. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed to the. — the fda's definition of a. Fda Definition Medical Device Manufacturer.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Fda Definition Medical Device Manufacturer Digital health center of excellence. — (o) manufacturer means any person who designs, manufactures, fabricates, assembles, or processes a. Determine if your product meets the definition of a medical device per section 201 (h) of the food, drug & cosmetic act. — medical devices are classified both by medical specialty (i.e., classification panels) and by the risk posed. Fda Definition Medical Device Manufacturer.