Medical Device Servicing Fda . — the food and drug administration (fda or agency) is announcing the availability of a final. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. Digital health center of excellence. — many medical devices are reused; fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). For instance, infant warmers, ventilators, endoscopes and defibrillators,. approvals and clearances, information on medical devices by type. — medical device manufacturers are required to comply with fda’s quality system. (a) where servicing is a specified requirement, each manufacturer shall establish and.
from www.youtube.com
fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). (a) where servicing is a specified requirement, each manufacturer shall establish and. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. Digital health center of excellence. — many medical devices are reused; For instance, infant warmers, ventilators, endoscopes and defibrillators,. — the food and drug administration (fda or agency) is announcing the availability of a final. approvals and clearances, information on medical devices by type. — medical device manufacturers are required to comply with fda’s quality system.
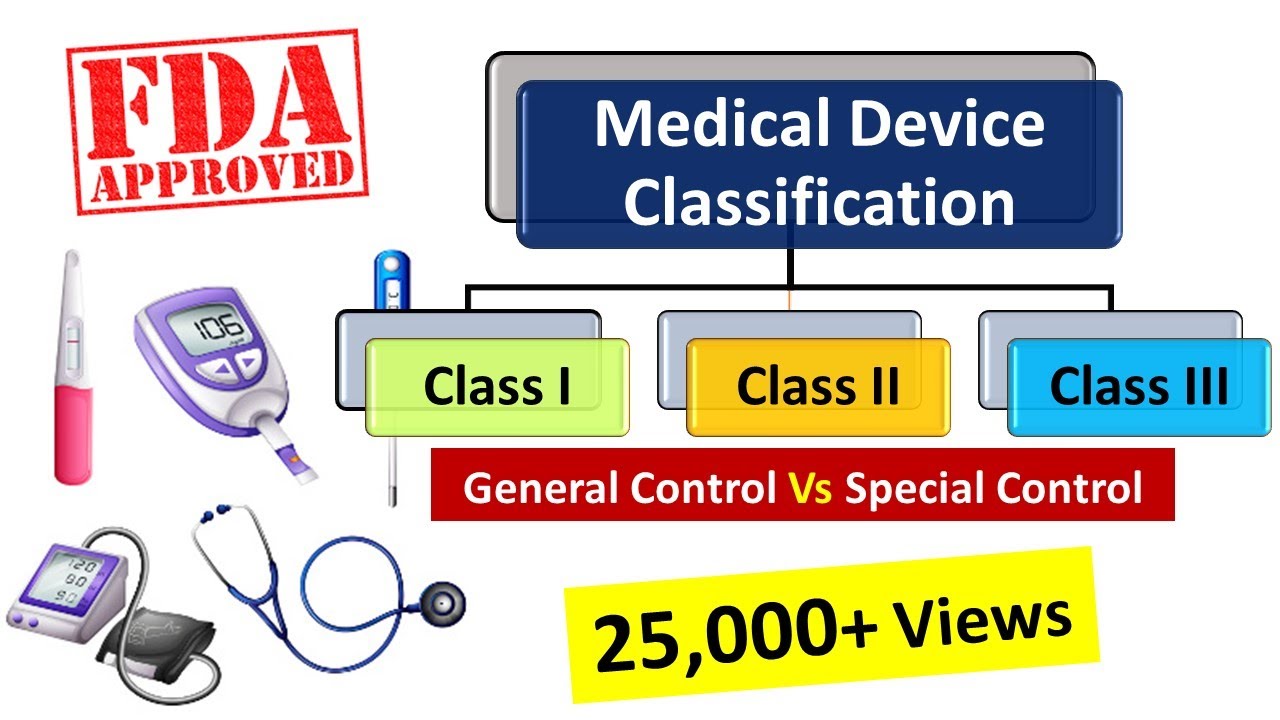
Medical Devices classification as per FDA Medical Device Regulations
Medical Device Servicing Fda — many medical devices are reused; approvals and clearances, information on medical devices by type. Digital health center of excellence. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. For instance, infant warmers, ventilators, endoscopes and defibrillators,. — many medical devices are reused; fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). — the food and drug administration (fda or agency) is announcing the availability of a final.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Medical Device Servicing Fda (a) where servicing is a specified requirement, each manufacturer shall establish and. For instance, infant warmers, ventilators, endoscopes and defibrillators,. — medical device manufacturers are required to comply with fda’s quality system. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. approvals and clearances, information on medical devices by. Medical Device Servicing Fda.
From medium.com
Get FDA Registration Certificate and FDA Renewal Service to Your Medical Device Servicing Fda For instance, infant warmers, ventilators, endoscopes and defibrillators,. — medical device manufacturers are required to comply with fda’s quality system. (a) where servicing is a specified requirement, each manufacturer shall establish and. Digital health center of excellence. approvals and clearances, information on medical devices by type. fda’s report on the quality, safety, and effectiveness of servicing of. Medical Device Servicing Fda.
From healthtrustpg.com
FDA Approval Update HealthTrust Performance Improvement For Healthcare Medical Device Servicing Fda For instance, infant warmers, ventilators, endoscopes and defibrillators,. — many medical devices are reused; Digital health center of excellence. (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. — section 710 of fdara required fda to issue a report on the continued. Medical Device Servicing Fda.
From deviceservicingalliance.com
Alliance Comments to FDA on Cybersecurity of Medical Devices Device Medical Device Servicing Fda — medical device manufacturers are required to comply with fda’s quality system. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). (a) where servicing is a specified requirement, each manufacturer shall. Medical Device Servicing Fda.
From www.rimsys.io
FDA listed, cleared, approved, granted what do these mean, and what’s Medical Device Servicing Fda — medical device manufacturers are required to comply with fda’s quality system. For instance, infant warmers, ventilators, endoscopes and defibrillators,. approvals and clearances, information on medical devices by type. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. (a) where servicing is a specified requirement, each manufacturer shall establish. Medical Device Servicing Fda.
From www.compliance-insight.com
The FDA Is Adopting ISO 13485 What This Means for Medical Devices Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. approvals and clearances, information on medical devices by type. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. (a) where servicing is a specified requirement, each manufacturer shall establish and. fda’s report. Medical Device Servicing Fda.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Medical Device Servicing Fda — many medical devices are reused; (a) where servicing is a specified requirement, each manufacturer shall establish and. For instance, infant warmers, ventilators, endoscopes and defibrillators,. approvals and clearances, information on medical devices by type. — medical device manufacturers are required to comply with fda’s quality system. Digital health center of excellence. fda’s report on the. Medical Device Servicing Fda.
From deviceservicingalliance.com
Alliance Comments to FDA on Cybersecurity of Medical Devices Device Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. For instance, infant warmers, ventilators, endoscopes and defibrillators,. Digital health center of excellence. — medical device manufacturers are required to comply with fda’s quality system. approvals and clearances, information on medical devices by type. (a) where servicing is a specified requirement,. Medical Device Servicing Fda.
From vem-medical.com
Medical Device Manufacturing Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. — many medical devices are reused; fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). Digital health center of excellence. — section 710 of fdara required fda to issue a report. Medical Device Servicing Fda.
From www.advamed.org
ThirdParty Servicing of Medical Devices AdvaMed Medical Device Servicing Fda — medical device manufacturers are required to comply with fda’s quality system. approvals and clearances, information on medical devices by type. (a) where servicing is a specified requirement, each manufacturer shall establish and. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. For instance, infant warmers, ventilators, endoscopes and. Medical Device Servicing Fda.
From www.youtube.com
Medical Device Servicing Community FDA Industry Updates YouTube Medical Device Servicing Fda approvals and clearances, information on medical devices by type. (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. — many medical devices are reused; — section 710 of fdara required fda to issue a report on the continued quality, safety, and.. Medical Device Servicing Fda.
From info.atlantisworldwide.com
New FDA Report Brings Good News for Medical Device Service Medical Device Servicing Fda — medical device manufacturers are required to comply with fda’s quality system. Digital health center of excellence. — many medical devices are reused; approvals and clearances, information on medical devices by type. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). For instance, infant warmers, ventilators, endoscopes. Medical Device Servicing Fda.
From www.scribd.com
FDA Report On Medical Device Servicing Rightly Recognizes Need For Medical Device Servicing Fda — medical device manufacturers are required to comply with fda’s quality system. Digital health center of excellence. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. — many medical devices are reused; For instance, infant warmers, ventilators, endoscopes and defibrillators,. (a) where servicing is a specified requirement, each manufacturer. Medical Device Servicing Fda.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Medical Device Servicing Fda approvals and clearances, information on medical devices by type. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). Digital health center of excellence. — many medical devices are reused; (a). Medical Device Servicing Fda.
From meddev-info.blogspot.com
Medical Device Regulation Basics US FDA Medical Device Classification Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. Digital health center of excellence. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). For instance, infant warmers, ventilators, endoscopes and defibrillators,. approvals and clearances, information on medical devices by type. . Medical Device Servicing Fda.
From medicaldevice510k.com
Guidelines Medicaldevice510k Medical Device Servicing Fda fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). (a) where servicing is a specified requirement, each manufacturer shall establish and. Digital health center of excellence. — medical device manufacturers are required to comply with fda’s quality system. For instance, infant warmers, ventilators, endoscopes and defibrillators,. approvals and. Medical Device Servicing Fda.
From remmed.com
FDA Registered Medical Devices What to Expect Remington Medical Device Servicing Fda — many medical devices are reused; — medical device manufacturers are required to comply with fda’s quality system. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). — the food and drug administration (fda or agency) is announcing the availability of a final. Digital health center of. Medical Device Servicing Fda.
From www.orielstat.com
How FDA Distinguishes Servicing from Remanufacturing a Medical Device Medical Device Servicing Fda For instance, infant warmers, ventilators, endoscopes and defibrillators,. — medical device manufacturers are required to comply with fda’s quality system. — many medical devices are reused; — the food and drug administration (fda or agency) is announcing the availability of a final. (a) where servicing is a specified requirement, each manufacturer shall establish and. approvals and. Medical Device Servicing Fda.
From synectic.net
Medical Device FDA Regulations Infographic Synectic Medical Device Servicing Fda approvals and clearances, information on medical devices by type. — medical device manufacturers are required to comply with fda’s quality system. — the food and drug administration (fda or agency) is announcing the availability of a final. — many medical devices are reused; — section 710 of fdara required fda to issue a report on. Medical Device Servicing Fda.
From www.vrogue.co
A Guide To Fda Design Controls For Your Medical Devic vrogue.co Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. approvals and clearances, information on medical devices by type. — many medical devices are reused; . Medical Device Servicing Fda.
From fdareporter.com
AI MEDICAL SERVICE INC. Announces FDA Breakthrough Device Designation Medical Device Servicing Fda approvals and clearances, information on medical devices by type. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). — the food and drug administration (fda or agency) is announcing the availability of a final. (a) where servicing is a specified requirement, each manufacturer shall establish and. —. Medical Device Servicing Fda.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. — many medical devices are reused; (a) where servicing is a specified requirement, each manufacturer shall establish and. fda’s report on the quality,. Medical Device Servicing Fda.
From www.simplerqms.com
Medical Device Classification (FDA & EU MDR) SimplerQMS Medical Device Servicing Fda — many medical devices are reused; For instance, infant warmers, ventilators, endoscopes and defibrillators,. — medical device manufacturers are required to comply with fda’s quality system. (a) where servicing is a specified requirement, each manufacturer shall establish and. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). . Medical Device Servicing Fda.
From www.greenlight.guru
What is the FDA Medical Device Registration Process? Medical Device Servicing Fda fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). — the food and drug administration (fda or agency) is announcing the availability of a final. Digital health center of excellence. (a) where servicing is a specified requirement, each manufacturer shall establish and. — many medical devices are reused;. Medical Device Servicing Fda.
From www.memsjournal.com
MEMS Journal The Largest MEMS Publication in the World BioMEMS Medical Device Servicing Fda (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). — section 710 of fdara required fda to issue a report on the continued quality,. Medical Device Servicing Fda.
From www.slideserve.com
PPT Crossing the Threshold ( FDA Regulatory Requirements for Medical Medical Device Servicing Fda Digital health center of excellence. — many medical devices are reused; approvals and clearances, information on medical devices by type. (a) where servicing is a specified requirement, each manufacturer shall establish and. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). — medical device manufacturers are required. Medical Device Servicing Fda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. — many medical devices are reused; For instance, infant warmers, ventilators, endoscopes and defibrillators,. (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. Digital health center. Medical Device Servicing Fda.
From fr.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Device Servicing Fda — medical device manufacturers are required to comply with fda’s quality system. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). For instance, infant warmers, ventilators, endoscopes and defibrillators,. approvals and clearances, information on medical devices by type. — the food and drug administration (fda or agency). Medical Device Servicing Fda.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Medical Device Servicing Fda — the food and drug administration (fda or agency) is announcing the availability of a final. Digital health center of excellence. — medical device manufacturers are required to comply with fda’s quality system. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). — section 710 of fdara. Medical Device Servicing Fda.
From blog.novasyte.com
FDA Confirms ThirdParty Entities Provide High Quality, Safe and Medical Device Servicing Fda (a) where servicing is a specified requirement, each manufacturer shall establish and. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. approvals and clearances, information on medical devices by type. — the food and drug administration (fda or agency) is announcing the availability of a final. — medical. Medical Device Servicing Fda.
From www.pinterest.com
Infographic on Understanding FDA Device Classes from Medical Device Servicing Fda — section 710 of fdara required fda to issue a report on the continued quality, safety, and. — many medical devices are reused; (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. — the food and drug administration (fda or agency). Medical Device Servicing Fda.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Device Servicing Fda — section 710 of fdara required fda to issue a report on the continued quality, safety, and. (a) where servicing is a specified requirement, each manufacturer shall establish and. For instance, infant warmers, ventilators, endoscopes and defibrillators,. — medical device manufacturers are required to comply with fda’s quality system. — the food and drug administration (fda or. Medical Device Servicing Fda.
From www.meddevicecorp.com
US FDA Registration Service of Food, Drugs, Medical Devices Medical Device Servicing Fda For instance, infant warmers, ventilators, endoscopes and defibrillators,. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). Digital health center of excellence. (a) where servicing is a specified requirement, each manufacturer shall establish and. — medical device manufacturers are required to comply with fda’s quality system. — section. Medical Device Servicing Fda.
From www.swisstechnologiesne.com
Medical Devices and The FDA Medical Device Servicing Fda (a) where servicing is a specified requirement, each manufacturer shall establish and. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda report on device servicing). Digital health center of excellence. — many medical devices are reused; approvals and clearances, information on medical devices by type. — section 710 of fdara required. Medical Device Servicing Fda.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Device Servicing Fda approvals and clearances, information on medical devices by type. Digital health center of excellence. — section 710 of fdara required fda to issue a report on the continued quality, safety, and. (a) where servicing is a specified requirement, each manufacturer shall establish and. fda’s report on the quality, safety, and effectiveness of servicing of medical devices (fda. Medical Device Servicing Fda.