Bromine Lose Gain Electrons . For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. Define the two types of ions. Ions are formed when atoms gain or lose electrons. We can see that the bromine has gained electrons, so it has been reduced. The charge of bromine changes depending on how it reacts with other elements. A cation (positively charged ion) forms when one or more electrons are removed from a. The iodide ions have lost electrons , so they have. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Atoms gain or lose electrons to form anions or cations, respectively.
from studylib.net
Ions are formed when atoms gain or lose electrons. The iodide ions have lost electrons , so they have. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. A cation (positively charged ion) forms when one or more electrons are removed from a. Define the two types of ions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms gain or lose electrons to form anions or cations, respectively. The charge of bromine changes depending on how it reacts with other elements. We can see that the bromine has gained electrons, so it has been reduced. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron.
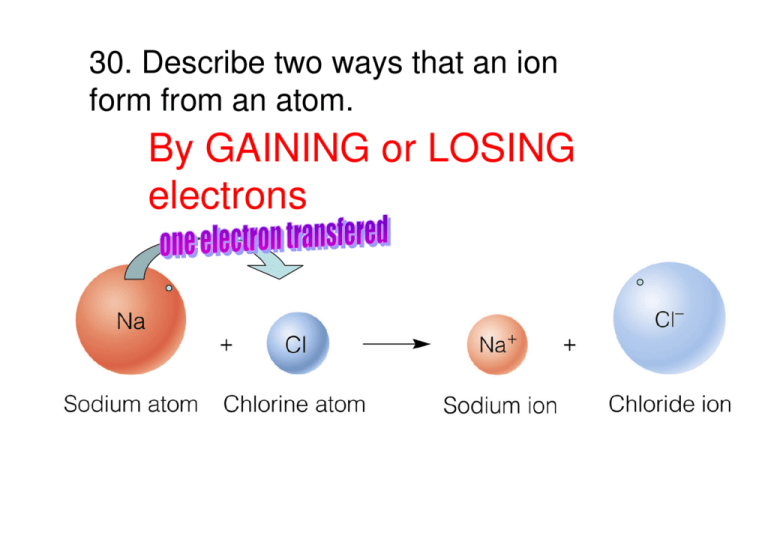
By GAINING or LOSING electrons
Bromine Lose Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. The iodide ions have lost electrons , so they have. A cation (positively charged ion) forms when one or more electrons are removed from a. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Define the two types of ions. We can see that the bromine has gained electrons, so it has been reduced. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. The charge of bromine changes depending on how it reacts with other elements. Atoms gain or lose electrons to form anions or cations, respectively. Ions are formed when atoms gain or lose electrons.
From slideplayer.com
Notes Ionic Bonds 1. Key Concept Ionic bonds form when electrons are Bromine Lose Gain Electrons Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. The charge of bromine changes depending on how it reacts with other elements. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Ions are formed when atoms gain or lose electrons. A. Bromine Lose Gain Electrons.
From www.vrogue.co
Oxidation Reduction Definition Reaction Examples Priy vrogue.co Bromine Lose Gain Electrons The charge of bromine changes depending on how it reacts with other elements. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms. Bromine Lose Gain Electrons.
From studylib.net
By GAINING or LOSING electrons Bromine Lose Gain Electrons Define the two types of ions. The charge of bromine changes depending on how it reacts with other elements. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. The iodide ions have lost electrons , so they have. Atoms that lose electrons acquire a positive charge as a. Bromine Lose Gain Electrons.
From www.numerade.com
Complete the following table to predict whether the given atom will Bromine Lose Gain Electrons For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. We can see that the bromine has gained electrons, so it has been reduced. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Ions are formed when atoms gain or lose. Bromine Lose Gain Electrons.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Bromine Lose Gain Electrons For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. We can see that the bromine has gained electrons, so it has been reduced. The charge of. Bromine Lose Gain Electrons.
From dokumen.tips
(PDF) WS, Ionic Bonding Edl Bonding WS Element Gain or Lose of Bromine Lose Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. The iodide ions have lost electrons , so they have. The charge of bromine changes depending on how it reacts with other elements. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36. Bromine Lose Gain Electrons.
From socratic.org
Why does fluorine have a higher ionization energy than bromine? Socratic Bromine Lose Gain Electrons The charge of bromine changes depending on how it reacts with other elements. A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Atoms that lose electrons acquire a positive charge as a result because they are left with. Bromine Lose Gain Electrons.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free Bromine Lose Gain Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. Ions are formed when atoms gain or lose electrons. The charge of bromine changes depending on how it reacts with other elements. Ions form when atoms lose or gain electrons close electron. Bromine Lose Gain Electrons.
From www.periodictableprintable.com
Periodic Table Elements Lose Or Gain Electrons 2024 Periodic Table Bromine Lose Gain Electrons The iodide ions have lost electrons , so they have. Atoms gain or lose electrons to form anions or cations, respectively. A cation (positively charged ion) forms when one or more electrons are removed from a. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms that lose. Bromine Lose Gain Electrons.
From www.coursehero.com
[Solved] Neutral bromine (Br2) can gain two electrons to the Bromine Lose Gain Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Atoms gain or lose electrons to form anions or cations, respectively. Define the two types of. Bromine Lose Gain Electrons.
From valenceelectrons.com
Complete Electron Configuration for Bromine (Br, Br ion) Bromine Lose Gain Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. A cation (positively charged ion) forms when one or more. Bromine Lose Gain Electrons.
From sarai-kobrien.blogspot.com
Metals Tend to Lose Electrons to Positive Ions Bromine Lose Gain Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms gain or lose electrons to form anions or cations, respectively. The iodide ions have lost electrons , so they have. Ions are formed when atoms gain or lose electrons. Define the two types of ions. Atoms that lose electrons acquire a positive charge as. Bromine Lose Gain Electrons.
From www.nagwa.com
Question Video Identifying the Arrows That Indicate Losing and Gaining Bromine Lose Gain Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. We can see that the bromine has gained electrons, so. Bromine Lose Gain Electrons.
From www.slideshare.net
10 28 How Many Electrons Do Atoms Gain Lose Bromine Lose Gain Electrons Ions are formed when atoms gain or lose electrons. We can see that the bromine has gained electrons, so it has been reduced. A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance. Bromine Lose Gain Electrons.
From www.teachoo.com
Elements P, Q, R & S have atomic numbers 11, 15, 17 & 18 respectively Bromine Lose Gain Electrons Define the two types of ions. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Ions are formed when atoms gain or lose electrons. The charge of bromine changes depending on how it reacts with other elements. A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms. Bromine Lose Gain Electrons.
From www.numerade.com
SOLVED Examining your labeled periodic table, which of the following Bromine Lose Gain Electrons Atoms gain or lose electrons to form anions or cations, respectively. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative.. Bromine Lose Gain Electrons.
From utedzz.blogspot.com
Periodic Table Fluorine Valence Electrons Periodic Table Timeline Bromine Lose Gain Electrons Ions are formed when atoms gain or lose electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. We can see that the bromine has gained electrons, so it has been reduced. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to. Bromine Lose Gain Electrons.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromine Lose Gain Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms gain or lose electrons to form anions or cations, respectively. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. We can see that the bromine has gained electrons, so it has been. Bromine Lose Gain Electrons.
From wordwall.net
Gain or Lose Electrons? Group sort Bromine Lose Gain Electrons Ions are formed when atoms gain or lose electrons. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Ions form when atoms lose or gain. Bromine Lose Gain Electrons.
From brainly.ph
Materials Rubbed Together Material Charge of which would the material Bromine Lose Gain Electrons The charge of bromine changes depending on how it reacts with other elements. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Define the two types of ions. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. Ions are formed when. Bromine Lose Gain Electrons.
From www.showme.com
Noble Gas Electron Configurations Electron Configuration, High School Bromine Lose Gain Electrons Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms gain or lose electrons to form anions or cations, respectively. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. We can see that the bromine. Bromine Lose Gain Electrons.
From stock.adobe.com
Br Bromine Element Information Facts, Properties, Trends, Uses and Bromine Lose Gain Electrons We can see that the bromine has gained electrons, so it has been reduced. Define the two types of ions. Ions are formed when atoms gain or lose electrons. The iodide ions have lost electrons , so they have. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative.. Bromine Lose Gain Electrons.
From www.numerade.com
SOLVED For the Bromine atom a) Determine the total number of unpaired Bromine Lose Gain Electrons Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. The charge of bromine changes depending on how it reacts with other elements. Atoms gain or lose electrons to form anions or cations, respectively. The iodide ions have lost electrons , so they have. Atoms that lose electrons acquire. Bromine Lose Gain Electrons.
From www.goodscience.com.au
Formation of Ions and Ionic Compounds Good Science Bromine Lose Gain Electrons The charge of bromine changes depending on how it reacts with other elements. Ions are formed when atoms gain or lose electrons. We can see that the bromine has gained electrons, so it has been reduced. A cation (positively charged ion) forms when one or more electrons are removed from a. For example, the neutral bromine atom, with 35 protons. Bromine Lose Gain Electrons.
From tech.noakmech.com
How Many Electrons Does Aluminum Need To Be Stable ZTech Bromine Lose Gain Electrons For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Define the two types of ions. We can see that the bromine has gained electrons, so it. Bromine Lose Gain Electrons.
From exofobtvx.blob.core.windows.net
Does Lead Lose Electrons at Howard Krause blog Bromine Lose Gain Electrons The iodide ions have lost electrons , so they have. A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms gain or lose electrons to form anions or cations, respectively. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive. Bromine Lose Gain Electrons.
From www.animalia-life.club
Electron Configuration For Bromine Bromine Lose Gain Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. We can see that the bromine has gained electrons, so it has been reduced. Define the two types of ions. Atoms that lose electrons acquire a positive charge as. Bromine Lose Gain Electrons.
From www.sciencecoverage.com
How Many Valence Electrons Does Bromine (Br) Have? [Valency of Bromine] Bromine Lose Gain Electrons The charge of bromine changes depending on how it reacts with other elements. A cation (positively charged ion) forms when one or more electrons are removed from a. Ions are formed when atoms gain or lose electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms that. Bromine Lose Gain Electrons.
From www.numerade.com
SOLVED Select the number of electrons that each atom needs t0 gain Bromine Lose Gain Electrons A cation (positively charged ion) forms when one or more electrons are removed from a. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. We can see that the bromine has gained electrons, so it has been reduced. Define the two types of ions. Atoms tend to lose,. Bromine Lose Gain Electrons.
From www.numerade.com
SOLVED 0 / 1 pts Question 11 As per octet rule, bromine atom would Bromine Lose Gain Electrons The charge of bromine changes depending on how it reacts with other elements. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of. Bromine Lose Gain Electrons.
From brennan-bogspotberry.blogspot.com
Does Selenium Gain or Lose Electrons Bromine Lose Gain Electrons Define the two types of ions. The charge of bromine changes depending on how it reacts with other elements. Ions are formed when atoms gain or lose electrons. Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron. Bromine Lose Gain Electrons.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN Bromine Lose Gain Electrons Atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged electrons to balance the positive charges of the protons. A cation (positively charged ion) forms when one or more electrons are removed from a. Ions are formed when atoms gain or lose electrons. Define the two types of ions. For example,. Bromine Lose Gain Electrons.
From www.numerade.com
SOLVED Neutral bromine (Br2) can gain two electrons to the Bromine Lose Gain Electrons We can see that the bromine has gained electrons, so it has been reduced. The iodide ions have lost electrons , so they have. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms that lose electrons acquire a positive charge as a result because they are left. Bromine Lose Gain Electrons.
From www.vrogue.co
Calculate The Number Of Electrons Lost Or Gained Duri vrogue.co Bromine Lose Gain Electrons Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Define the two types of ions. A cation (positively charged ion) forms when one or more electrons are removed from a. The iodide ions have lost electrons , so they have. Atoms tend to lose, gain, or share some. Bromine Lose Gain Electrons.
From socratic.org
How can you tell if an element wants to gain or lose electrons? Socratic Bromine Lose Gain Electrons Atoms tend to lose, gain, or share some valance electrons, making bonds to acquire the electron. The charge of bromine changes depending on how it reacts with other elements. A cation (positively charged ion) forms when one or more electrons are removed from a. Atoms that lose electrons acquire a positive charge as a result because they are left with. Bromine Lose Gain Electrons.