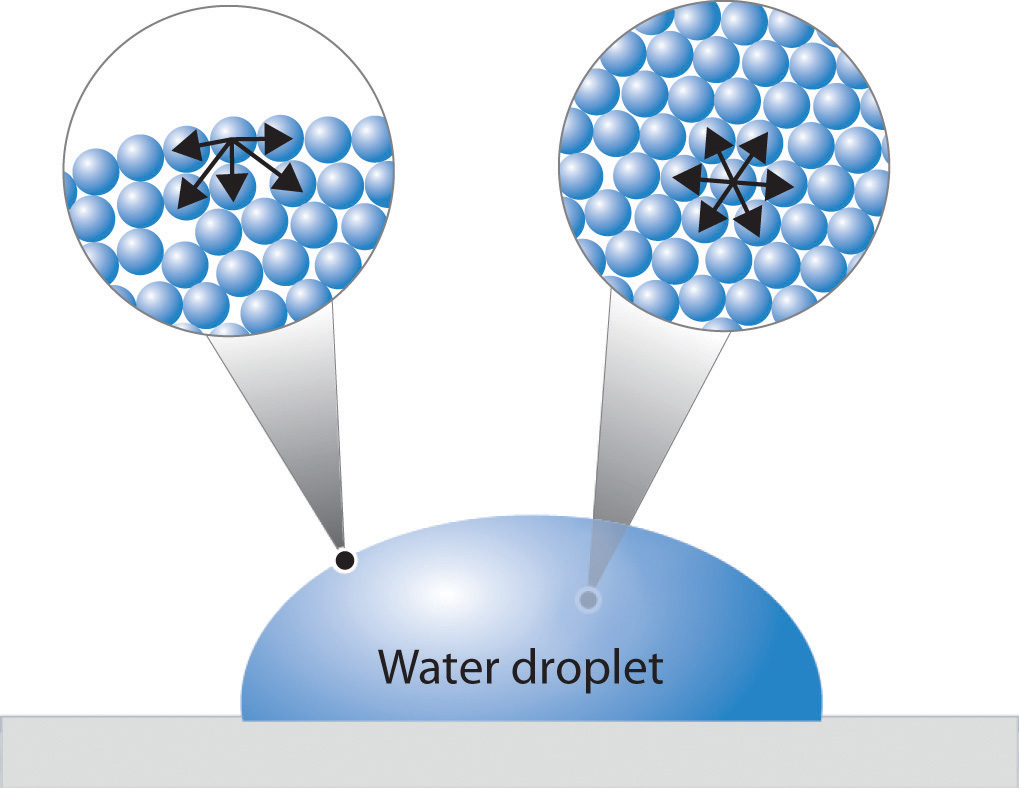
Does Surface Tension Affect The Density Column . the project concluded that surface tension in each liquid is dependent on the nature of the liquid. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. After noting the density of each. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. surface tension (γ γ) can be measured by capillary action of the liquid, as. surface tension is the energy required to increase the surface area of a liquid by a given amount. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ.
from chem.libretexts.org
surface tension is the energy required to increase the surface area of a liquid by a given amount. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. After noting the density of each. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. surface tension (γ γ) can be measured by capillary action of the liquid, as. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced.
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts
Does Surface Tension Affect The Density Column the project concluded that surface tension in each liquid is dependent on the nature of the liquid. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. surface tension (γ γ) can be measured by capillary action of the liquid, as. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. surface tension is the energy required to increase the surface area of a liquid by a given amount. After noting the density of each. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced.
From giofsecel.blob.core.windows.net
What Properties Does Surface Tension Itself Affect at Jon blog Does Surface Tension Affect The Density Column After noting the density of each. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid by a given amount. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ.. Does Surface Tension Affect The Density Column.
From hxezuywoi.blob.core.windows.net
Surface Tension Of Liquid Zinc at Matthew Woodland blog Does Surface Tension Affect The Density Column the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. After noting the density of each. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. . Does Surface Tension Affect The Density Column.
From www.reddit.com
Determination of Surface Tension Read Chemistry r/ChemistryTeachers Does Surface Tension Affect The Density Column cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. surface tension (γ γ) can be measured by capillary action of the liquid, as. with application of the antonoff rule the “solidliquid” interface. Does Surface Tension Affect The Density Column.
From fyozfaljz.blob.core.windows.net
How Does Surface Tension Affect Humans at Andrew Rex blog Does Surface Tension Affect The Density Column Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. the project concluded that surface tension in each liquid is dependent on the. Does Surface Tension Affect The Density Column.
From www.mr-cfd.com
Surface Tension Effect on Air Bubbles under Water Column, Ansys Fluent Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. the project concluded that surface tension in each liquid is dependent on the nature of the liquid.. Does Surface Tension Affect The Density Column.
From www.merchantnavydecoded.com
What is Surface tension? Why does surface tension occur? Merchant Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid. Does Surface Tension Affect The Density Column.
From classnotes.org.in
Surface Tension Chemistry, Class 11, States of Matter Does Surface Tension Affect The Density Column Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. After noting the density of each. surface tension (γ γ) can be measured by capillary action of the liquid, as. the project concluded. Does Surface Tension Affect The Density Column.
From www.scribd.com
How Does Surface Tension Affect Energy Release Rate of Cracks PDF Does Surface Tension Affect The Density Column cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is the energy required to increase the surface area of a liquid by a given amount. surface tension (γ γ) can be measured by capillary action of the liquid, as. with application of the antonoff rule. Does Surface Tension Affect The Density Column.
From gioenbudo.blob.core.windows.net
How Does Surface Tension Affect The Functioning Of Living Organisms at Does Surface Tension Affect The Density Column cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. surface tension (γ γ) can be measured by capillary action of the liquid, as. Γ = hρg r 2cosθ γ = h. Does Surface Tension Affect The Density Column.
From askfilo.com
Surface tension does not vary with Filo Does Surface Tension Affect The Density Column the project concluded that surface tension in each liquid is dependent on the nature of the liquid. surface tension is the energy required to increase the surface area of a liquid by a given amount. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced.. Does Surface Tension Affect The Density Column.
From chem.libretexts.org
Chapter 7.3 Unique Properties of Liquids Chemistry LibreTexts Does Surface Tension Affect The Density Column the project concluded that surface tension in each liquid is dependent on the nature of the liquid. surface tension is the energy required to increase the surface area of a liquid by a given amount. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the. Does Surface Tension Affect The Density Column.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL Does Surface Tension Affect The Density Column After noting the density of each. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. . Does Surface Tension Affect The Density Column.
From fyozfaljz.blob.core.windows.net
How Does Surface Tension Affect Humans at Andrew Rex blog Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the extent of. Does Surface Tension Affect The Density Column.
From gioenbudo.blob.core.windows.net
How Does Surface Tension Affect The Functioning Of Living Organisms at Does Surface Tension Affect The Density Column After noting the density of each. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. surface tension (γ γ) can be measured by capillary action of the liquid, as. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface tension is. Does Surface Tension Affect The Density Column.
From exyeqkqyy.blob.core.windows.net
How Does Surface Tension Affect Droplet Size at Joseph Carpenter blog Does Surface Tension Affect The Density Column Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. surface tension (γ γ) can be measured by capillary action of the liquid, as. After noting the density of each. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the. Does Surface Tension Affect The Density Column.
From giojkmyma.blob.core.windows.net
How Does Surface Tension Force Water To Curve at Linda Robles blog Does Surface Tension Affect The Density Column the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. with application of the antonoff rule the “solidliquid” interface tension. Does Surface Tension Affect The Density Column.
From upberi.com
Surface Tension Definition, Formula, Causes, Examples, and FAQs (2023) Does Surface Tension Affect The Density Column the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. Γ = hρg r 2cosθ γ = h ρ g r 2. Does Surface Tension Affect The Density Column.
From lessonlistdashboards.z13.web.core.windows.net
What Is The Density Of Salt Water Does Surface Tension Affect The Density Column the project concluded that surface tension in each liquid is dependent on the nature of the liquid. surface tension (γ γ) can be measured by capillary action of the liquid, as. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid. Does Surface Tension Affect The Density Column.
From gioenbudo.blob.core.windows.net
How Does Surface Tension Affect The Functioning Of Living Organisms at Does Surface Tension Affect The Density Column Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. surface tension (γ γ) can be measured by capillary action of the liquid,. Does Surface Tension Affect The Density Column.
From giofsecel.blob.core.windows.net
What Properties Does Surface Tension Itself Affect at Jon blog Does Surface Tension Affect The Density Column with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. surface tension. Does Surface Tension Affect The Density Column.
From fyozfaljz.blob.core.windows.net
How Does Surface Tension Affect Humans at Andrew Rex blog Does Surface Tension Affect The Density Column surface tension (γ γ) can be measured by capillary action of the liquid, as. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid. Does Surface Tension Affect The Density Column.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit Does Surface Tension Affect The Density Column surface tension (γ γ) can be measured by capillary action of the liquid, as. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. surface tension is. Does Surface Tension Affect The Density Column.
From www.youtube.com
Surface tension and capillary rise YouTube Does Surface Tension Affect The Density Column the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. After noting the density of each. cohesive forces between molecules cause. Does Surface Tension Affect The Density Column.
From exyeqkqyy.blob.core.windows.net
How Does Surface Tension Affect Droplet Size at Joseph Carpenter blog Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. the. Does Surface Tension Affect The Density Column.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts Does Surface Tension Affect The Density Column with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. surface. Does Surface Tension Affect The Density Column.
From exyrndcaw.blob.core.windows.net
What Causes The Surface Tension Of Water at Olson blog Does Surface Tension Affect The Density Column surface tension (γ γ) can be measured by capillary action of the liquid, as. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. the extent of the rise (or fall). Does Surface Tension Affect The Density Column.
From exyrndcaw.blob.core.windows.net
What Causes The Surface Tension Of Water at Olson blog Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced.. Does Surface Tension Affect The Density Column.
From ar.inspiredpencil.com
Density Of Fluids Does Surface Tension Affect The Density Column surface tension (γ γ) can be measured by capillary action of the liquid, as. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. After noting the density of each. with application of. Does Surface Tension Affect The Density Column.
From www.chegg.com
Solved a) [20 points] Find the angle of contact in Figure 1 Does Surface Tension Affect The Density Column After noting the density of each. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. surface tension (γ γ) can be measured by capillary action of the liquid, as. surface tension is the energy required to increase the surface area of a liquid by. Does Surface Tension Affect The Density Column.
From fyozfaljz.blob.core.windows.net
How Does Surface Tension Affect Humans at Andrew Rex blog Does Surface Tension Affect The Density Column After noting the density of each. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. Γ = hρg r 2cosθ γ = h ρ g r 2 c o s θ. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx). Does Surface Tension Affect The Density Column.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. the project concluded that surface tension in each liquid is dependent on the nature of the liquid. After noting the density of each. the extent of the rise (or fall) is directly proportional to the surface tension of the. Does Surface Tension Affect The Density Column.
From exoxqkwlq.blob.core.windows.net
How Does Surface Tension Relate To Water at Ron Lowery blog Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. surface tension (γ γ) can be measured by capillary action of the liquid, as. Γ = hρg. Does Surface Tension Affect The Density Column.
From www.chegg.com
Solved 3. In Fig.P.3 both fluids are at 20°C. If surface Does Surface Tension Affect The Density Column cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. After noting the density of each. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. the project concluded that surface tension in each liquid is dependent. Does Surface Tension Affect The Density Column.
From giojkmyma.blob.core.windows.net
How Does Surface Tension Force Water To Curve at Linda Robles blog Does Surface Tension Affect The Density Column cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. the extent of the rise (or fall) is directly proportional to the surface tension of the liquid and inversely proportional to the density of the liquid and the radius. surface tension is the energy required to increase the surface. Does Surface Tension Affect The Density Column.
From www.researchgate.net
Variations of the film thickness with different surface tension Does Surface Tension Affect The Density Column surface tension is the energy required to increase the surface area of a liquid by a given amount. with application of the antonoff rule the “solidliquid” interface tension gradient along the column, (dγ * sl / dx) can be replaced. the project concluded that surface tension in each liquid is dependent on the nature of the liquid.. Does Surface Tension Affect The Density Column.