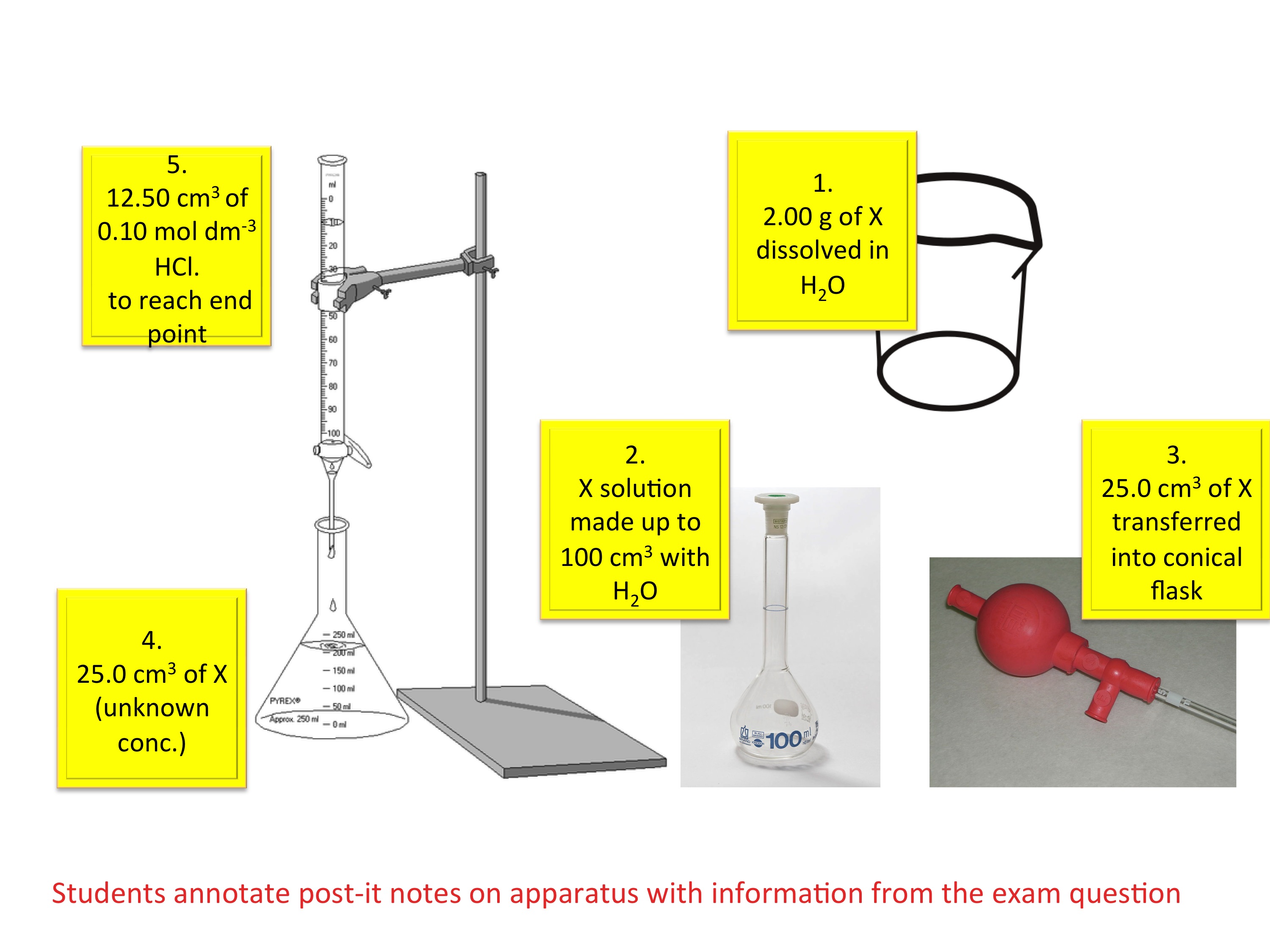
Titration Experiment Formula . What are titrant and analyte. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. the amount of added titrant is determined from its concentration and volume: in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. What is the equivalence point of a titration. Learn the titration graph and titration equation.
from thescienceteacher.co.uk
N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. Learn the titration graph and titration equation. What is the equivalence point of a titration. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the amount of added titrant is determined from its concentration and volume: What are titrant and analyte.
Concentration and titrations teaching resources the science teacher
Titration Experiment Formula the amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. What is the equivalence point of a titration. What are titrant and analyte. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the amount of added titrant is determined from its concentration and volume: in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: Learn the titration graph and titration equation. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Experiment Formula N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. the amount of added titrant is determined from its concentration and volume: What is the equivalence point of a titration. Learn the titration graph and titration equation. in this experiment, a technique known as a titration will be. Titration Experiment Formula.
From fyomgpmqq.blob.core.windows.net
Titration Calculations Exam Questions at Bree Duckett blog Titration Experiment Formula the amount of added titrant is determined from its concentration and volume: in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. What are titrant and analyte. What is the equivalence point of a titration. Learn the titration graph and titration equation. the above. Titration Experiment Formula.
From gioxrzofe.blob.core.windows.net
Calculation Formula For Titration at Allison Rosario blog Titration Experiment Formula in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. What is the equivalence point of a titration. What are titrant and analyte. when doing titration calculations,. Titration Experiment Formula.
From dxogpmtvr.blob.core.windows.net
Titration Experiment Grade 12 Memo 2022 Pdf at Micheal Berg blog Titration Experiment Formula when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3. Titration Experiment Formula.
From www.chegg.com
Solved Experiment 11, Titrations Report Sheet Titration Experiment Formula the amount of added titrant is determined from its concentration and volume: the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Learn the titration graph. Titration Experiment Formula.
From studylib.net
EXPERIMENT 3. ACIDBASE TITRATIONS DETERMINATION OF Titration Experiment Formula What is the equivalence point of a titration. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. N (mol) = c (mol /l) * v (l) and the amount of. Titration Experiment Formula.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Experiment Formula the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Learn the titration graph and titration equation. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. the amount of added titrant is determined. Titration Experiment Formula.
From www.tessshebaylo.com
Karl Fischer Titration Equation Tessshebaylo Titration Experiment Formula the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. What is the equivalence point of a titration. Learn the titration graph and titration equation. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. N (mol). Titration Experiment Formula.
From childhealthpolicy.vumc.org
🐈 Titration experiment results. How do you report a titration Titration Experiment Formula What are titrant and analyte. Learn the titration graph and titration equation. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. What is the equivalence point of a titration. the amount of added titrant is determined from its concentration and volume: N (mol) = c (mol. Titration Experiment Formula.
From theedge.com.hk
Chemistry How To Titration The Edge Titration Experiment Formula N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. What is the equivalence point of a titration. What are titrant and analyte. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Learn the titration graph and. Titration Experiment Formula.
From www.tes.com
Acid Base Titration Theory, Titration Lab, Titration Stoichiometry Titration Experiment Formula in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the amount of added titrant is determined from its concentration and volume: Learn the titration graph and titration equation. What is the equivalence point of a titration. N (mol) = c (mol /l) * v (l) and. Titration Experiment Formula.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Experiment Formula the amount of added titrant is determined from its concentration and volume: What is the equivalence point of a titration. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Learn the titration graph and titration equation. in this experiment, a technique known as. Titration Experiment Formula.
From fyoyxafat.blob.core.windows.net
Weak Acid Strong Base Titration Lab Report Discussion at Elizabeth Titration Experiment Formula in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Learn the titration graph and titration equation. N (mol) = c (mol /l) *. Titration Experiment Formula.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration Experiment Formula in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: Learn the titration graph and titration equation. the amount of added titrant is determined from its concentration. Titration Experiment Formula.
From exywypgkb.blob.core.windows.net
Redox Titration A Level Chemistry Practical at Charles Lum blog Titration Experiment Formula What are titrant and analyte. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. Learn the titration graph and titration equation. the amount of. Titration Experiment Formula.
From nl.vecteezy.com
zuur baseren titratie experiment en fasen van kleur verandering Titration Experiment Formula What is the equivalence point of a titration. Learn the titration graph and titration equation. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. the above. Titration Experiment Formula.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration Experiment Formula What are titrant and analyte. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: the amount of added titrant is determined from its concentration and volume: in a titration,. Titration Experiment Formula.
From www.ck12.org
Titration (Calculations) Overview ( Video ) Chemistry CK12 Titration Experiment Formula the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. What are titrant and analyte. the amount of added titrant is determined from its concentration and volume: Learn the titration graph and titration equation. when doing titration calculations, there are 4 main “regions” of the titration, that. Titration Experiment Formula.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Experiment Formula in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Learn the titration graph and titration equation. What are titrant and analyte. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. What is. Titration Experiment Formula.
From mungfali.com
Acid Base Titration Procedure Titration Experiment Formula What are titrant and analyte. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: What is the equivalence point of a titration. Learn the titration graph and titration equation.. Titration Experiment Formula.
From exybsrswn.blob.core.windows.net
Redox Titration Kmno4 at Amanda Young blog Titration Experiment Formula when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of. Titration Experiment Formula.
From www.youtube.com
Redox Titration Example Question 2 (Easy) ALevel Chemistry YouTube Titration Experiment Formula What is the equivalence point of a titration. What are titrant and analyte. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in a titration, 25.00. Titration Experiment Formula.
From exyxqrdzm.blob.core.windows.net
Chemistry Titration Indicators at Richard Veit blog Titration Experiment Formula when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Learn the titration graph and titration equation. N (mol) = c (mol /l) * v (l) and the amount of. Titration Experiment Formula.
From www.studypool.com
SOLUTION Titration Chemistry Class 11th Studypool Titration Experiment Formula N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. in this experiment, a technique known as a titration will be used to determine the. Titration Experiment Formula.
From cebvudsb.blob.core.windows.net
Titration Standard Formula at Megan Snead blog Titration Experiment Formula What is the equivalence point of a titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: Learn the titration graph and titration equation. the amount of added titrant. Titration Experiment Formula.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration Experiment Formula when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. What are titrant and analyte. Learn the titration graph and titration equation. the amount of added titrant is determined from its. Titration Experiment Formula.
From www.savemyexams.com
Titrations (1.7.2) Edexcel A Level Chemistry Revision Notes 2017 Titration Experiment Formula Learn the titration graph and titration equation. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. the amount of added titrant is determined from its concentration and volume: What is the equivalence point of a titration. the above equation works only for neutralizations in which there is. Titration Experiment Formula.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Titration Experiment Formula What is the equivalence point of a titration. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. the amount of added titrant is. Titration Experiment Formula.
From mmerevise.co.uk
Titrations and Uncertainties MME Titration Experiment Formula Learn the titration graph and titration equation. the amount of added titrant is determined from its concentration and volume: N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the. Titration Experiment Formula.
From giogrwbcc.blob.core.windows.net
Acidity Titration Formula at Kimberly Carroll blog Titration Experiment Formula the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: Learn the titration graph and titration equation. in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution. Titration Experiment Formula.
From www.aiophotoz.com
Difference Between Redox Titration And Acid Base Titration Images Titration Experiment Formula in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the amount of added titrant is determined from its concentration and volume: What are titrant and analyte. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual.. Titration Experiment Formula.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Experiment Formula in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used. Titration Experiment Formula.
From www.vrogue.co
Acid Base Titration Formula vrogue.co Titration Experiment Formula the amount of added titrant is determined from its concentration and volume: What are titrant and analyte. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: in a titration, 25.00 cm 3 of 0.200 mol/dm 3 sodium hydroxide solution is exactly neutralised by 22.70 cm 3 of a dilute.. Titration Experiment Formula.
From www.numerade.com
SOLVED In a particular titration experiment a 25.0 mL sample of an Titration Experiment Formula Learn the titration graph and titration equation. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. when doing titration calculations, there are 4 main “regions” of the titration, that correspond to calculation strategies: What are titrant and analyte. N (mol) = c (mol /l) * v (l). Titration Experiment Formula.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Experiment Formula the amount of added titrant is determined from its concentration and volume: Learn the titration graph and titration equation. N (mol) = c (mol /l) * v (l) and the amount of titrant can be used in the usual. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. Titration Experiment Formula.