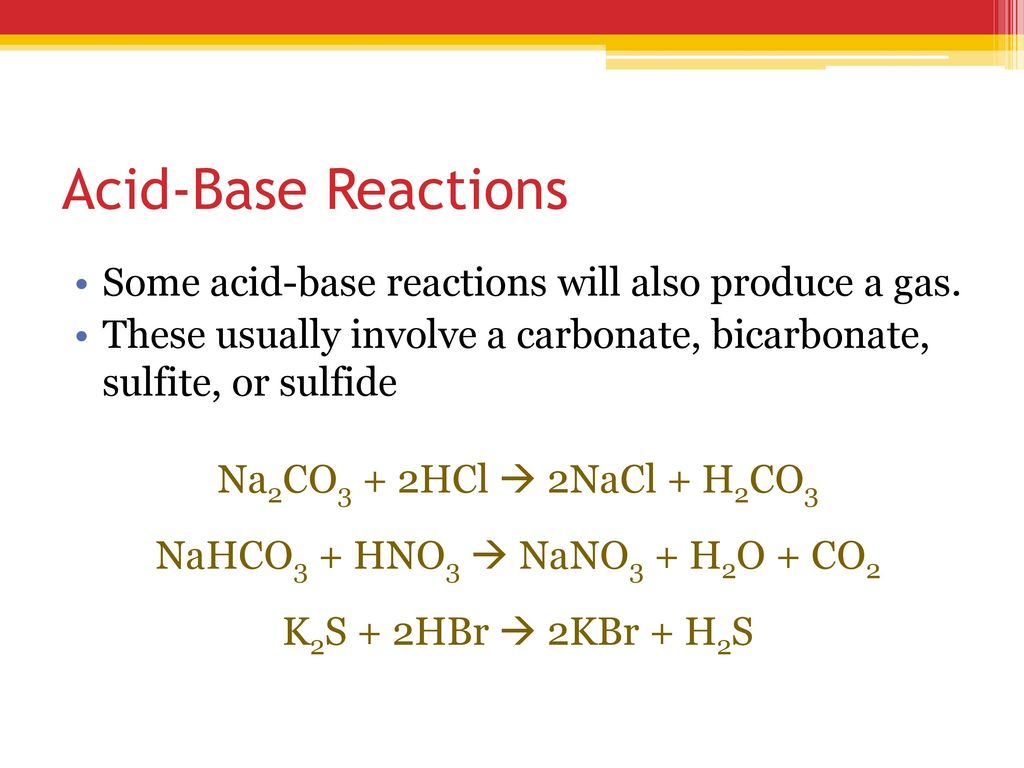
The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is . This page assumes that you know about ph curves for all the. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: For example, the reaction of. Complex redox reactions, especially in acidic or basic solutions. Acid plus base yields water plus salt. Updated on november 04, 2019. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate.
from slideplayer.com
Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Updated on november 04, 2019. For example, the reaction of. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: One mole of sodium carbonate [na 2 co 3] and two moles of nitric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. This page assumes that you know about ph curves for all the. Acid plus base yields water plus salt. Complex redox reactions, especially in acidic or basic solutions.
Reactions in Aqueous Solutions and Stiochiometry ppt download
The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: For example, the reaction of. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Complex redox reactions, especially in acidic or basic solutions. This page assumes that you know about ph curves for all the. Updated on november 04, 2019. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. Acid plus base yields water plus salt.
From slideplayer.com
Classification and Balancing of Chemical Reactions ppt download The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is This page assumes that you know about ph curves for all the. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Updated on november 04, 2019. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From mavink.com
Acid Base Reaction Chart The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Complex redox reactions, especially in acidic or basic solutions. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. Acid plus base yields water plus salt. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The graph shows the results obtained. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.numerade.com
SOLVED Cu(s) + HNO3(aq) → Cu(NO3)2(aq) + H2O(l) + NO2(g) A redox reaction Cu(NO3)2(aq The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. This page assumes that you know about ph curves for all the. For example, the reaction of. Updated on november 04, 2019. Acid plus base yields water plus salt. If the base is. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideshare.net
Topic 08 introduction The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Complex redox reactions, especially in acidic or basic solutions. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Acid plus base yields water plus salt. Updated on november 04, 2019. This page assumes that you know about ph curves for all the. One mole of sodium carbonate [na 2 co 3] and two moles of nitric.. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Complex redox reactions, especially in acidic or basic solutions. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Acid plus base yields water plus salt. For example, the reaction of. Updated on november 04, 2019. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. This page assumes that you know about. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideserve.com
PPT The pH scale PowerPoint Presentation, free download ID4827855 The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Updated on november 04, 2019. Complex redox reactions, especially in acidic or basic solutions. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. One mole of sodium carbonate. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.youtube.com
How to Balance Na2CO3 + HNO3 = NaNO3 + H2O + CO2 YouTube The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Acid plus base yields water plus salt. For example, the reaction of. Updated on november 04, 2019. One mole of sodium carbonate [na 2 co 3] and two moles of nitric.. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From slideplayer.com
Reactions in Aqueous Solutions and Stiochiometry ppt download The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is This page assumes that you know about ph curves for all the. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Acid plus base yields water plus salt. Updated on november 04, 2019. One mole of sodium carbonate [na 2 co 3] and two moles. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideserve.com
PPT AcidBase Reactions PowerPoint Presentation, free download ID3969900 The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is This page assumes that you know about ph curves for all the. Acid plus base yields water plus salt. Complex redox reactions, especially in acidic or basic solutions. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is For example, the reaction of. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Complex redox reactions, especially in acidic or basic solutions. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. This page assumes that you know about ph curves. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Acid plus base yields water plus salt. For example, the reaction of. Complex redox reactions, especially in acidic or basic solutions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.youtube.com
Type of Reaction for NaHCO3 = Na2CO3 + H2O + CO2 YouTube The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is For example, the reaction of. Complex redox reactions, especially in acidic or basic solutions. Acid plus base yields water plus salt. This page assumes that you know about ph curves for all the. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From byjus.com
in the titration of Na2CO3 by HCl using methyl orange indicator, thevolume required at the The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Acid plus base yields water plus salt. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. Updated on november 04, 2019. Complex redox reactions, especially in. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Complex redox reactions, especially in acidic or basic solutions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Updated on november 04, 2019. Acid plus base yields water plus salt. One mole of sodium carbonate [na 2 co 3] and. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.studypool.com
SOLUTION Acid base indicators Studypool The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is One mole of sodium carbonate [na 2 co 3] and two moles of nitric. For example, the reaction of. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Sodium carbonate + nitric acid = carbon dioxide + water + sodium. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is One mole of sodium carbonate [na 2 co 3] and two moles of nitric. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.researchgate.net
Reaction pathway of Na2CO3carbon in an inert atmosphere (a); and... Download Scientific Diagram The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. This page assumes that you know about. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.youtube.com
Na2CO3 + HCl Sodium Carbonate + Hydrochloric Acid YouTube The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Updated on november 04, 2019. Acid plus base yields water plus salt. Complex redox reactions, especially in acidic or basic solutions. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The graph. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.researchgate.net
Acidbase indicators and their parameters. Download Table The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is For example, the reaction of. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. This page assumes that you know about ph curves for all the. One mole of sodium carbonate [na 2 co 3] and two moles of nitric.. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is This page assumes that you know about ph curves for all the. Updated on november 04, 2019. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Acid plus base yields water plus. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. This page assumes that you know about ph curves for all the. Acid plus base yields water plus salt. For example, the reaction of. Complex redox reactions, especially in acidic or basic solutions. One mole of sodium carbonate [na 2 co 3] and two moles of nitric.. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. This page assumes that you know about ph curves for all the. For example, the reaction of.. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.numerade.com
SOLVED For the acidbase reaction of sodium bicarbonate, NaHCO3, with hydrochloric acid, HCl The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Complex redox reactions, especially in acidic or basic solutions. This page assumes that you know about ph curves for all the. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Acid plus base yields water plus salt. For example, the reaction of. If the base is a metal hydroxide, then the general formula for the reaction. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideserve.com
PPT BASES PowerPoint Presentation, free download ID4980324 The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Complex redox reactions, especially in acidic or basic solutions. Acid plus base yields water plus salt. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Updated on november 04, 2019. For example,. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideserve.com
PPT Acidbase titrations PowerPoint Presentation, free download ID4401802 The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Updated on november 04, 2019. This page assumes that you know about ph curves for all the. For example, the reaction of. Complex redox reactions, especially in acidic or basic solutions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid.. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.researchgate.net
Characteristics of the acidbase indicators Download Table The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Complex redox reactions, especially in acidic or basic solutions. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Acid plus base yields water plus salt. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. One mole. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From slideplayer.com
Volumetric Analysis Using titration of a solution with known concentration to determine The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: One mole of sodium carbonate [na 2 co 3] and two moles of nitric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID5368605 The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Updated on november 04, 2019. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. Acid plus base yields water plus salt. Complex redox reactions, especially in. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.numerade.com
SOLVED The balanced equation for the reaction between sodium carbonate and hydrochloric acid is The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is One mole of sodium carbonate [na 2 co 3] and two moles of nitric. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Complex redox reactions, especially in acidic or basic solutions. For example, the reaction of. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideserve.com
PPT Introduction to Acids and Bases PowerPoint Presentation, free download ID2220867 The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. This page assumes that you know about ph curves. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From topblogtenz.com
Is Na2CO3 an acid or base or salt? Strong or Weak Sodium carbonate The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Acid plus base yields water plus salt. One mole of sodium carbonate [na 2 co 3] and two moles of nitric. The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Updated on november 04, 2019. This page assumes that you. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From scienceinfo.com
AcidBase Reaction (Neutralization Reaction) The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Updated on november 04, 2019. This page assumes that you know about ph curves for all the. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: One mole of sodium carbonate [na 2 co 3] and two moles of nitric. For example, the reaction of.. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From learningpectose.z13.web.core.windows.net
How To Write Acid Base Reactions The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: The graph shows the results obtained using two indicators (methyl red and phenolphthalein) for the titration of 0.100 m solutions of a strong acid (hcl) and a weak acid. Sodium carbonate + nitric acid = carbon. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.youtube.com
ACID BASE TITRATION IN BANGAL, অ্যাসিড ক্ষার টাইট্রেশন, Na2CO3 vs HCl YouTube The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is Acid plus base yields water plus salt. If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: This page assumes that you know about ph curves for all the. Complex redox reactions, especially in acidic or basic solutions. The graph shows the results obtained using two. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.
From www.slideserve.com
PPT Theoretical Yield Which Reactant is Limiting? PowerPoint Presentation ID705844 The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is If the base is a metal hydroxide, then the general formula for the reaction of an acid with a base is described as follows: One mole of sodium carbonate [na 2 co 3] and two moles of nitric. Updated on november 04, 2019. Sodium carbonate + nitric acid = carbon dioxide + water + sodium nitrate. Acid plus base yields. The Best Indicator For An Acid Base Reaction Between Na2Co3 And Hno3 Is.