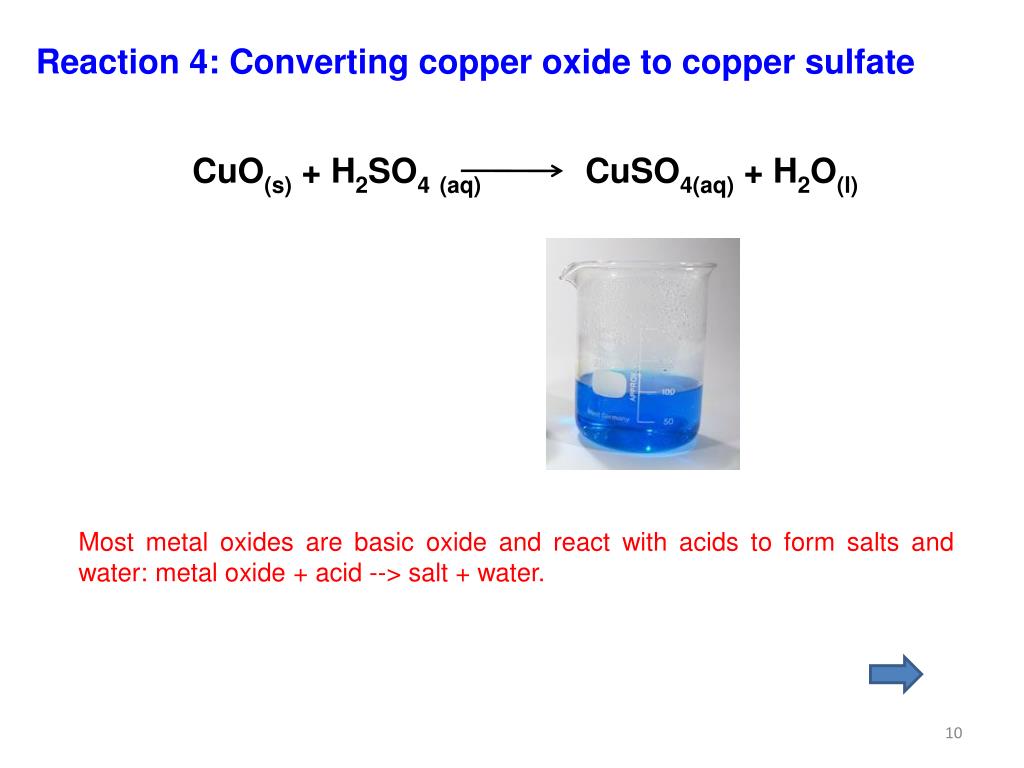
Copper Oxide Vs Hydroxide . Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Cuprous oxide is very well known as a. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Find out how to use the reaction to determine the concentration of. Copper is still oxidized by forming these substances. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. It's copper carbonate hydroxide or hydrated copper chloride.
from www.slideserve.com
The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Find out how to use the reaction to determine the concentration of. Cuprous oxide is very well known as a. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Copper is still oxidized by forming these substances. It's copper carbonate hydroxide or hydrated copper chloride. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts.
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint
Copper Oxide Vs Hydroxide Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Copper is still oxidized by forming these substances. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. It's copper carbonate hydroxide or hydrated copper chloride. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Cuprous oxide is very well known as a. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Find out how to use the reaction to determine the concentration of. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine.
From www.vrogue.co
Group 2 Metal Reactions A Level Chemistrystudent vrogue.co Copper Oxide Vs Hydroxide The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Copper is still oxidized by forming these substances. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Find out how to use the reaction to determine the concentration of. Learn about the. Copper Oxide Vs Hydroxide.
From www.youtube.com
Make Copper II Oxide, and Copper II Hydroxide YouTube Copper Oxide Vs Hydroxide Cuprous oxide is very well known as a. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Find out how to use the reaction to determine the concentration of. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Copper is. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper Oxide Vs Hydroxide It's copper carbonate hydroxide or hydrated copper chloride. Find out how to use the reaction to determine the concentration of. Copper is still oxidized by forming these substances. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Cuprous oxide is very well known as a. The idea is that. Copper Oxide Vs Hydroxide.
From www.youtube.com
3. Copper Hydroxide to Copper Oxide (Cu Again Lab) YouTube Copper Oxide Vs Hydroxide Cuprous oxide is very well known as a. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. It's copper carbonate hydroxide or hydrated copper chloride. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Learn about the different types of copper compounds,. Copper Oxide Vs Hydroxide.
From www.youtube.com
Making Copper Hydroxide YouTube Copper Oxide Vs Hydroxide It's copper carbonate hydroxide or hydrated copper chloride. Find out how to use the reaction to determine the concentration of. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to. Copper Oxide Vs Hydroxide.
From www.learnatnoon.com
What are the three types of oxides? Noon Academy Copper Oxide Vs Hydroxide Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Cuprous oxide is very well known as a. Find out how to use the reaction to determine the concentration of. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states. Copper Oxide Vs Hydroxide.
From www.researchgate.net
1 The distribution of arsenite and thioarsenic species in a reducing... Download Scientific Copper Oxide Vs Hydroxide Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. It's copper carbonate hydroxide or hydrated copper chloride. Cuprous oxide is very well known as a. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. The idea is that copper nitrate is converted to. Copper Oxide Vs Hydroxide.
From pubs.acs.org
Activation of Oxygen and Hydrogen Peroxide by Copper(II) Coupled with Hydroxylamine for Copper Oxide Vs Hydroxide The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. It's copper carbonate hydroxide or hydrated copper chloride. Find out how to use the reaction to determine the concentration. Copper Oxide Vs Hydroxide.
From www.researchgate.net
Deconvoluted (a) Cu 2p3/2 and (b) O 1s XPS spectra of copper oxide... Download Scientific Diagram Copper Oxide Vs Hydroxide Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. It's copper carbonate hydroxide or hydrated copper chloride. Copper is still oxidized by forming these substances. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. The idea is. Copper Oxide Vs Hydroxide.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II oxide YouTube Copper Oxide Vs Hydroxide Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. It's copper carbonate hydroxide or hydrated copper chloride. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most. Copper Oxide Vs Hydroxide.
From www.differencebetween.com
Difference Between Copper Hydroxide and Copper Oxychloride Compare the Difference Between Copper Oxide Vs Hydroxide Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Find out how to use the reaction to determine the concentration of. Copper is still oxidized by forming these substances. It's copper carbonate hydroxide or hydrated copper chloride. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation, free download ID35476 Copper Oxide Vs Hydroxide Cuprous oxide is very well known as a. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Find out how to use the reaction to determine the concentration of. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. It's copper carbonate hydroxide. Copper Oxide Vs Hydroxide.
From www.differencebetween.com
Difference Between Copper Hydroxide and Copper Oxychloride Compare the Difference Between Copper Oxide Vs Hydroxide It's copper carbonate hydroxide or hydrated copper chloride. Copper is still oxidized by forming these substances. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Find out how to use the reaction to determine the concentration of. Cuprous oxide is very well known as a. Learn how to. Copper Oxide Vs Hydroxide.
From thecontentauthority.com
Hydroxide vs Oxide Fundamental Differences Of These Terms Copper Oxide Vs Hydroxide Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Copper is still oxidized by forming these substances. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Learn about the reactions of copper (ii) and. Copper Oxide Vs Hydroxide.
From ar.inspiredpencil.com
Copper Oxidation Copper Oxide Vs Hydroxide Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Copper is still oxidized by forming these substances. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Learn about the properties, occurrence, extraction and reactions of copper, a. Copper Oxide Vs Hydroxide.
From www.youtube.com
How to Write the Formula for Copper (II) hydroxide YouTube Copper Oxide Vs Hydroxide Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Cuprous oxide is very well known as a. Find out how to use the reaction to determine the concentration of. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Copper is still oxidized by forming these substances.. Copper Oxide Vs Hydroxide.
From www.youtube.com
Copper Hydroxide Synthesis YouTube Copper Oxide Vs Hydroxide It's copper carbonate hydroxide or hydrated copper chloride. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Cuprous oxide is very well known as a. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Copper is still oxidized by forming. Copper Oxide Vs Hydroxide.
From www.science-revision.co.uk
Displacement reactions Copper Oxide Vs Hydroxide The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Copper is still oxidized by forming these substances. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Cuprous oxide is very well known as a. Learn about the reactions of copper (ii). Copper Oxide Vs Hydroxide.
From meghachem.org
Types of copper oxide and it’s uses Copper Oxide Vs Hydroxide Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Types Of Copper Oxide And Its Uses PowerPoint Presentation, free download ID11565338 Copper Oxide Vs Hydroxide Cuprous oxide is very well known as a. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Learn about the properties, occurrence, extraction and reactions of copper, a. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Predicting Reactions PowerPoint Presentation, free download ID4182778 Copper Oxide Vs Hydroxide It's copper carbonate hydroxide or hydrated copper chloride. Cuprous oxide is very well known as a. Find out how to use the reaction to determine the concentration of. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Learn about the different types of copper compounds,. Copper Oxide Vs Hydroxide.
From byjus.com
What is diference between base,basic oxide and basic hydroxide? Copper Oxide Vs Hydroxide Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Find out how to use the reaction to determine the concentration of. It's copper carbonate hydroxide or hydrated copper chloride. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality,. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Solubility of metal hydroxides, and amphoteric behavior. PowerPoint Presentation ID381650 Copper Oxide Vs Hydroxide Copper is still oxidized by forming these substances. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Cuprous oxide is very well known as a. Learn about the reactions of. Copper Oxide Vs Hydroxide.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas YouTube Copper Oxide Vs Hydroxide The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Cuprous oxide is very well known as a. Learn about the reactions of copper (ii) and copper (i) ions. Copper Oxide Vs Hydroxide.
From www.youtube.com
Make Copper hydroxide, Copper carbonate and Copper oxide (Cu(OH)2, CuCO3 and CuO) YouTube Copper Oxide Vs Hydroxide Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Copper is still oxidized by forming these substances. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Learn about the properties, occurrence, extraction and reactions. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper Oxide Vs Hydroxide Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Cuprous oxide is very well known as a. Learn about the reactions of copper (ii) and. Copper Oxide Vs Hydroxide.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green chemistry route Bulletin Copper Oxide Vs Hydroxide It's copper carbonate hydroxide or hydrated copper chloride. Find out how to use the reaction to determine the concentration of. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Cuprous oxide is very well known as a. Learn about the properties, occurrence, extraction and reactions. Copper Oxide Vs Hydroxide.
From www.researchgate.net
Solid metal hydroxides, oxidehydroxides, oxides, and ionic species... Download Table Copper Oxide Vs Hydroxide Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Copper is still oxidized by forming these substances. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Learn how to name and write formulas for ionic, covalent, and acid compounds using. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper Oxide Vs Hydroxide Cuprous oxide is very well known as a. Copper is still oxidized by forming these substances. It's copper carbonate hydroxide or hydrated copper chloride. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine.. Copper Oxide Vs Hydroxide.
From www.ceramic-glazes.com
Copper Hydroxide Cupric hydroxide patina for ceramics Copper Oxide Vs Hydroxide Find out how to use the reaction to determine the concentration of. Cuprous oxide is very well known as a. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Learn about the. Copper Oxide Vs Hydroxide.
From www.youtube.com
Acidic and Basic Oxides and Hydroxides YouTube Copper Oxide Vs Hydroxide The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Copper is still oxidized by forming these substances. Learn how to name and write formulas for ionic, covalent, and acid compounds using the principles of charge neutrality, greek prefixes, and acid salts. Cuprous oxide is very well known as a. It's copper carbonate. Copper Oxide Vs Hydroxide.
From www.slideserve.com
PPT Lecture 4 Thorium Chemistry PowerPoint Presentation, free download ID6796887 Copper Oxide Vs Hydroxide Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Copper is still oxidized. Copper Oxide Vs Hydroxide.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water YouTube Copper Oxide Vs Hydroxide Find out how to use the reaction to determine the concentration of. Copper is still oxidized by forming these substances. Learn about the reactions of copper (ii) and copper (i) ions in solution, including the oxidation of iodide ions to iodine. Cuprous oxide is very well known as a. The idea is that copper nitrate is converted to hydroxide, oxide,. Copper Oxide Vs Hydroxide.
From blog.eoscu.com
Copper vs. Cuprous Oxide What is the Difference? Copper Oxide Vs Hydroxide Cuprous oxide is very well known as a. The idea is that copper nitrate is converted to hydroxide, oxide, sulfate and then finally solid copper. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal.. Copper Oxide Vs Hydroxide.
From www.shutterstock.com
Interaction Copper Oxide Hydrogen Stock Vector (Royalty Free) 1133976842 Shutterstock Copper Oxide Vs Hydroxide Cuprous oxide is very well known as a. Learn about the different types of copper compounds, especially cuprous and cupric, which are the most common oxidation states of. Learn about the properties, occurrence, extraction and reactions of copper, a malleable and conductive metal. Copper is still oxidized by forming these substances. It's copper carbonate hydroxide or hydrated copper chloride. The. Copper Oxide Vs Hydroxide.