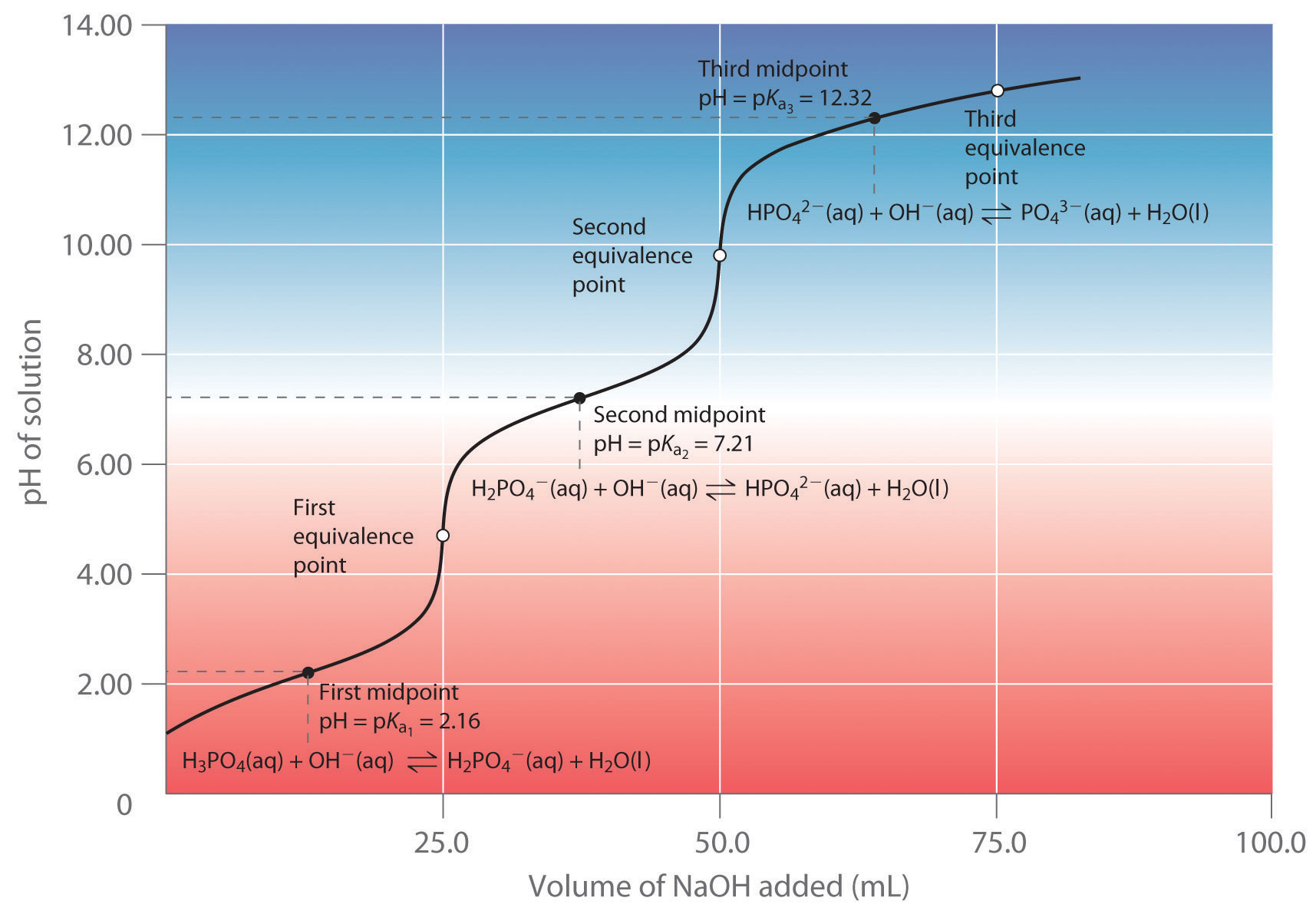
Equivalence Point Titration Temperature . sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a.
from saylordotorg.github.io
An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e.
AcidBase Titrations
Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration.
From www.numerade.com
SOLVED Consider the titration curve shown 0.250M HI is the titrant Equivalence Point Titration Temperature An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of. Equivalence Point Titration Temperature.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. the. Equivalence Point Titration Temperature.
From schoolbag.info
Titration and Buffers Acids and Bases Equivalence Point Titration Temperature the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. to evaluate the relationship between a titration’s equivalence point and its end point we need to. Equivalence Point Titration Temperature.
From exoaqbcwf.blob.core.windows.net
Equivalence Point Titration With Base at Alvin Barajas blog Equivalence Point Titration Temperature An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only. Equivalence Point Titration Temperature.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Equivalence Point Titration Temperature.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Equivalence Point Titration Temperature to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. the point of inflection (located. Equivalence Point Titration Temperature.
From quizspattering.z21.web.core.windows.net
How To Find Equivalence Point From Data Equivalence Point Titration Temperature the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. the point of inflection (located at the midpoint of the vertical part. Equivalence Point Titration Temperature.
From www.youtube.com
Thermometric Titration YouTube Equivalence Point Titration Temperature the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Equivalence Point Titration Temperature.
From exokpafwp.blob.core.windows.net
Titration Endpoint Calculator at Kevin Dowell blog Equivalence Point Titration Temperature the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a.. Equivalence Point Titration Temperature.
From ceckgokq.blob.core.windows.net
Titration Curve In Biochemistry at William Speece blog Equivalence Point Titration Temperature sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence. Equivalence Point Titration Temperature.
From solvedlib.com
Idenbfy the equivalence point 0n the titration curve … SolvedLib Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only. Equivalence Point Titration Temperature.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog Equivalence Point Titration Temperature the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a.. Equivalence Point Titration Temperature.
From dornshuld.chemistry.msstate.edu
5.10 Titration Curves Chemistry Equivalence Point Titration Temperature sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. the. Equivalence Point Titration Temperature.
From cevgzfch.blob.core.windows.net
Titration With Equivalence Point at Richard Patel blog Equivalence Point Titration Temperature the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. the equivalence. Equivalence Point Titration Temperature.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate. Equivalence Point Titration Temperature.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Equivalence Point Titration Temperature sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. the equivalence point is the point in a titration where the amount of titrant added is enough to. Equivalence Point Titration Temperature.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Equivalence Point Titration Temperature the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. to evaluate the relationship between a titration’s equivalence point and its end point. Equivalence Point Titration Temperature.
From exoliotyy.blob.core.windows.net
Equivalence Point Titration Example at Daniel Hoggard blog Equivalence Point Titration Temperature the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. it titration when all analyte has been consumed by the reaction, rate of. Equivalence Point Titration Temperature.
From quizspattering.z21.web.core.windows.net
How To Find Equivalence Point Of Titration Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. the equivalence point is the point in a titration where the amount of titrant added is enough to. Equivalence Point Titration Temperature.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Equivalence Point Titration Temperature to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. An increase or decrease determines the equivalence point and inflection in the temperature curve can be. Equivalence Point Titration Temperature.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Equivalence Point Titration Temperature the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e.. Equivalence Point Titration Temperature.
From dornshuld.chemistry.msstate.edu
5.10 Titration Curves Chemistry Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration.. Equivalence Point Titration Temperature.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point Titration Temperature the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a.. Equivalence Point Titration Temperature.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Equivalence Point Titration Temperature sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. An increase or decrease determines the equivalence point and inflection in the temperature curve can be. Equivalence Point Titration Temperature.
From solvedlib.com
What is a titration? What is the equivalence point o… SolvedLib Equivalence Point Titration Temperature An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence. Equivalence Point Titration Temperature.
From vasasimonmackenzie.blogspot.com
Equivalence Point Titration Simon Mackenzie Equivalence Point Titration Temperature to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration.. Equivalence Point Titration Temperature.
From exoapenql.blob.core.windows.net
Titration Curve Ka Value at Clarence Ellsworth blog Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence point for the titration. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. sketch out. Equivalence Point Titration Temperature.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. the. Equivalence Point Titration Temperature.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Equivalence Point Titration Temperature to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence. Equivalence Point Titration Temperature.
From www.numerade.com
SOLVED Consider the titration curve below for the titration of oxalic Equivalence Point Titration Temperature sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. to evaluate the relationship between a titration’s equivalence point and its. Equivalence Point Titration Temperature.
From studygripewater.z21.web.core.windows.net
How To Find Equivalence Point Equivalence Point Titration Temperature to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. sketch out a plot representing. Equivalence Point Titration Temperature.
From saylordotorg.github.io
AcidBase Titrations Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. the point of inflection (located at the midpoint of the vertical part of the curve) is the equivalence. Equivalence Point Titration Temperature.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Equivalence Point Titration Temperature it titration when all analyte has been consumed by the reaction, rate of temperature changes i.e. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of. Equivalence Point Titration Temperature.
From kunduz.com
[ANSWERED] reach the equivalence point Based on the titration curve it Equivalence Point Titration Temperature sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a reasonable approximation of the. it titration when all analyte has been consumed by the reaction,. Equivalence Point Titration Temperature.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Equivalence Point Titration Temperature An increase or decrease determines the equivalence point and inflection in the temperature curve can be observed. the equivalence point is the point in a titration where the amount of titrant added is enough to completely neutralize the analyte. to evaluate the relationship between a titration’s equivalence point and its end point we need to construct only a. Equivalence Point Titration Temperature.