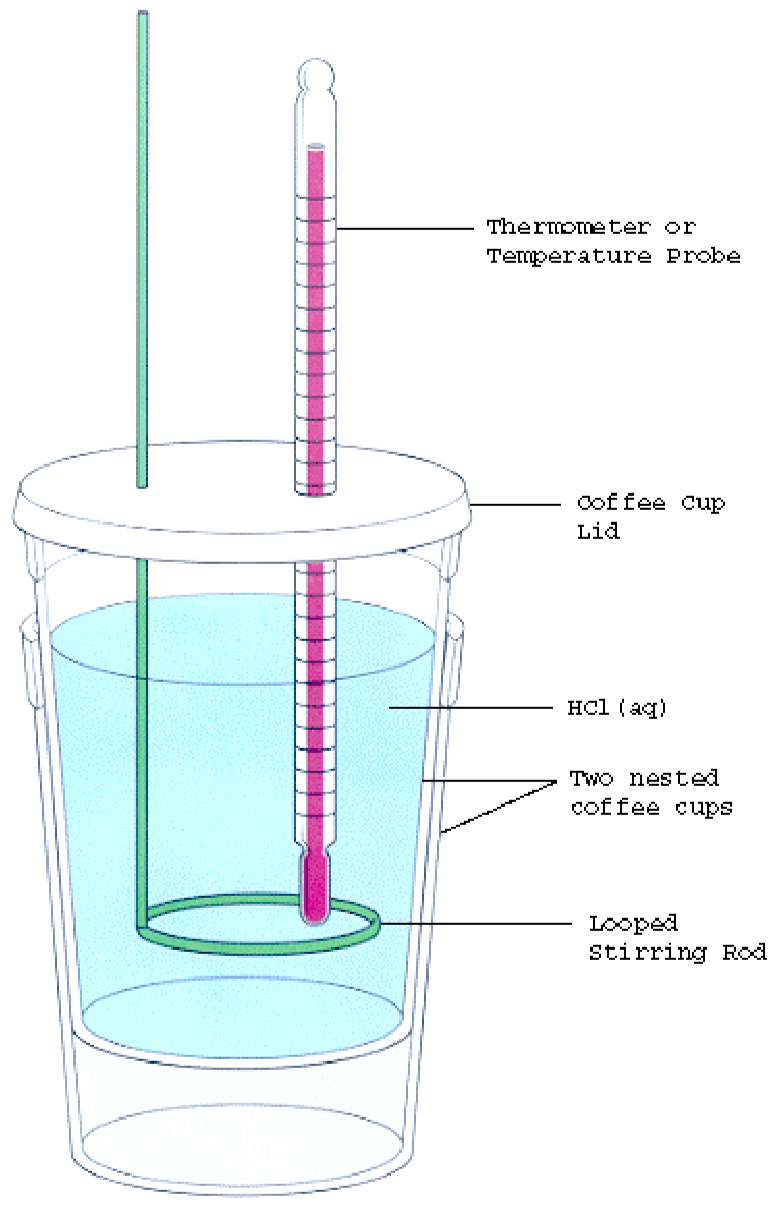
Calorimetry Experiment Diagram . The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry is used to measure amounts of heat transferred to. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The experiment will require two thermometers, one for the calorimeter and one for the. Obtain or assemble a calorimeter as shown in figure 9. Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. In a typical calorimetry experiment: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Determination of calorimeter constant 1.
from chem.libretexts.org
The experiment will require two thermometers, one for the calorimeter and one for the. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. In a typical calorimetry experiment: The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is used to measure amounts of heat transferred to. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Determination of calorimeter constant 1. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Obtain or assemble a calorimeter as shown in figure 9.
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Calorimetry Experiment Diagram The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In a typical calorimetry experiment: Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Determination of calorimeter constant 1. The experiment will require two thermometers, one for the calorimeter and one for the. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. Obtain or assemble a calorimeter as shown in figure 9.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Diagram Obtain or assemble a calorimeter as shown in figure 9. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. In a typical calorimetry experiment: Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Learn about calorimetry for. Calorimetry Experiment Diagram.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Experiment Diagram Obtain or assemble a calorimeter as shown in figure 9. In a typical calorimetry experiment: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions.. Calorimetry Experiment Diagram.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Experiment Diagram Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. Determination of calorimeter constant 1. The experiment will require two thermometers, one for the calorimeter and one for the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry.. Calorimetry Experiment Diagram.
From www.researchgate.net
Temperature measurements during a typical calorimetry experiment Calorimetry Experiment Diagram Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. Determination of calorimeter constant 1. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In a typical calorimetry experiment: Obtain or assemble a calorimeter as shown in figure. Calorimetry Experiment Diagram.
From www.animalia-life.club
Calorimeter Diagram Calorimetry Experiment Diagram Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure. Calorimetry Experiment Diagram.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Experiment Diagram Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. The experiment will require two thermometers, one for the calorimeter and one for the. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. These experiments often involve measuring. Calorimetry Experiment Diagram.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Experiment Diagram In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. These experiments often involve measuring the temperature changes that occur in. Calorimetry Experiment Diagram.
From wisc.pb.unizin.org
Calorimetry continued Types of Calorimeters and Analyzing Heat Flow Calorimetry Experiment Diagram In a typical calorimetry experiment: The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. The experiment will require two thermometers, one for the calorimeter and one for. Calorimetry Experiment Diagram.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry Experiment Diagram In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Obtain or assemble a calorimeter as shown in figure 9. The experiment will require two thermometers, one for the calorimeter and one for the. Calorimetry is the study of heat transferred in a. Calorimetry Experiment Diagram.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimetry Experiment Diagram In a typical calorimetry experiment: These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. Obtain or assemble a calorimeter as shown in figure 9. Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. The diagram on the right shows a very. Calorimetry Experiment Diagram.
From dxojkdaez.blob.core.windows.net
Calorimetry Physics Examples at Jeremy Wetmore blog Calorimetry Experiment Diagram Determination of calorimeter constant 1. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. The diagram on the right shows a very simple calorimetry experiment to. Calorimetry Experiment Diagram.
From joiaygdte.blob.core.windows.net
How To Use A Calorimeter Step By Step at Albert Jones blog Calorimetry Experiment Diagram Determination of calorimeter constant 1. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. The diagram on the right shows a very simple calorimetry. Calorimetry Experiment Diagram.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Calorimetry Experiment Diagram Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Determination of calorimeter constant 1. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is. Calorimetry Experiment Diagram.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Experiment Diagram Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. Calorimetry is used to measure amounts of heat transferred to. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. In a typical calorimetry experiment: In this experiment you will heat a known. Calorimetry Experiment Diagram.
From materialschreiner.z19.web.core.windows.net
What Is Calorimetry Give One Example Calorimetry Experiment Diagram Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning. Calorimetry Experiment Diagram.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Experiment Diagram One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In a typical calorimetry experiment: In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Obtain or assemble a calorimeter as. Calorimetry Experiment Diagram.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry Experiment Diagram One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Determination of calorimeter constant 1. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. Learn about calorimetry for igcse chemistry and how to determine energy changes. Calorimetry Experiment Diagram.
From surfguppy.com
Enthalpy Surfguppy Chemistry made easy visual learning Calorimetry Experiment Diagram Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. These experiments often involve measuring the temperature changes that occur in water or aqueous. Calorimetry Experiment Diagram.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Experiment Diagram In a typical calorimetry experiment: Obtain or assemble a calorimeter as shown in figure 9. Determination of calorimeter constant 1. Calorimetry is used to measure amounts of heat transferred to. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. The experiment will. Calorimetry Experiment Diagram.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Experiment Diagram The experiment will require two thermometers, one for the calorimeter and one for the. In a typical calorimetry experiment: Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Calorimetry is used to measure amounts of heat transferred to. Determination of calorimeter constant 1. Learn about calorimetry for. Calorimetry Experiment Diagram.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimetry Experiment Diagram Obtain or assemble a calorimeter as shown in figure 9. Determination of calorimeter constant 1. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. Simple calorimetry experiments can. Calorimetry Experiment Diagram.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Experiment Diagram The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions.. Calorimetry Experiment Diagram.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry Experiment Diagram One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. In a typical calorimetry experiment: The diagram on the right shows. Calorimetry Experiment Diagram.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry Experiment Diagram Determination of calorimeter constant 1. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Learn about calorimetry for igcse chemistry. Calorimetry Experiment Diagram.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Experiment Diagram Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Obtain or assemble a calorimeter as shown in figure 9. Learn about calorimetry for igcse chemistry and. Calorimetry Experiment Diagram.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry Experiment Diagram Determination of calorimeter constant 1. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Learn about calorimetry for igcse chemistry and how to determine energy changes in. Calorimetry Experiment Diagram.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Experiment Diagram Determination of calorimeter constant 1. These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. In a typical calorimetry experiment: Obtain or assemble a calorimeter as shown in figure 9. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. One. Calorimetry Experiment Diagram.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Calorimetry Experiment Diagram The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. The experiment will require two thermometers, one for the calorimeter and one for the. These experiments often involve. Calorimetry Experiment Diagram.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Experiment Diagram The experiment will require two thermometers, one for the calorimeter and one for the. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. In a typical calorimetry. Calorimetry Experiment Diagram.
From stock.adobe.com
Vettoriale Stock illustration of chemistry and physics, Calorimeter Calorimetry Experiment Diagram The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. In this experiment you will heat a known mass of a metal to a known temperature and then. Calorimetry Experiment Diagram.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Experiment Diagram Calorimetry is used to measure amounts of heat transferred to. The experiment will require two thermometers, one for the calorimeter and one for the. Determination of calorimeter constant 1. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Simple calorimetry experiments can be used to calculate the heat energy. Calorimetry Experiment Diagram.
From www.researchgate.net
Basic principle of isothermal titration calorimetry. Schematic Calorimetry Experiment Diagram In this experiment you will heat a known mass of a metal to a known temperature and then transfer it to a calorimeter that contains a known. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. These experiments often involve measuring the temperature changes that occur in water. Calorimetry Experiment Diagram.
From exonwzknq.blob.core.windows.net
Calorimetry Experiment Gcse Chemistry at Sanders blog Calorimetry Experiment Diagram These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. Simple calorimetry experiments can be used to calculate the heat energy transferred during reactions such as combustion, displacement, dissolving and neutralisation. The experiment will require two thermometers, one for the calorimeter and one for the. Calorimetry is used to measure amounts of. Calorimetry Experiment Diagram.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Experiment Diagram These experiments often involve measuring the temperature changes that occur in water or aqueous solutions during these reactions. The experiment will require two thermometers, one for the calorimeter and one for the. Calorimetry is the study of heat transferred in a chemical reaction, and a calorimeter is the tool used to measure this heat. Obtain or assemble a calorimeter as. Calorimetry Experiment Diagram.
From users.highland.edu
Calorimetry Calorimetry Experiment Diagram Determination of calorimeter constant 1. The diagram on the right shows a very simple calorimetry experiment to measure the heat energy released from burning fuel. Calorimetry is used to measure amounts of heat transferred to. Learn about calorimetry for igcse chemistry and how to determine energy changes in chemical reactions, including solution reactions and. In a typical calorimetry experiment: The. Calorimetry Experiment Diagram.