Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) . Only manganese dioxide gives a gaseous product. Manganese dioxide and copper (ii) oxide, (using. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. (i) when each of the compound is heated with conc. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: If no gas is evolved it is cuo. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) manganese dioxide and copper (ii). Study the flow chart and give balanced equations with conditions for the.
from www.slideserve.com
(i) when each of the compound is heated with conc. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. If no gas is evolved it is cuo. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. Distinguish between the following pairs of compounds using the reagent given in the bracket. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Manganese dioxide and copper (ii) oxide, (using.
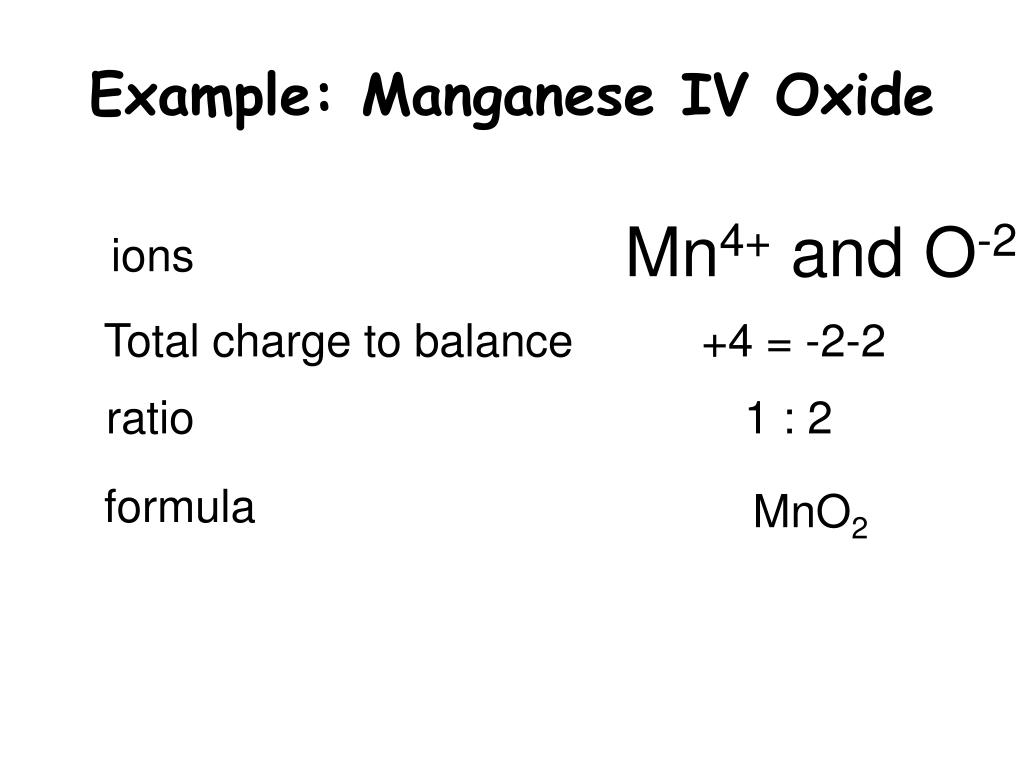
PPT Naming Multivalent Compounds PowerPoint Presentation, free
Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) (i) when each of the compound is heated with conc. (i) manganese dioxide and copper (ii). Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. (i) when each of the compound is heated with conc. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. If no gas is evolved it is cuo. Manganese dioxide and copper (ii) oxide, (using. Distinguish between the following pairs of compounds using the reagent given in the bracket. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Only manganese dioxide gives a gaseous product. Study the flow chart and give balanced equations with conditions for the. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2.
From segudanginformasibagus254.blogspot.com
Manganese Dioxide And Hydrogen Peroxide Equation / 0 1 3 0 4 1 0 Slajd Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Only manganese dioxide gives a gaseous product. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. If no gas is evolved it is cuo. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) If no gas is evolved it is cuo. Distinguish between the following pairs of compounds using the reagent given in the bracket. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. (i) manganese dioxide and copper (ii). Study the flow chart and give balanced equations with. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From estudiar.informacion.my.id
Cu Hcl Estudiar Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. (i) manganese dioxide and copper (ii). Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: If no gas is evolved it is cuo. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.numerade.com
SOLVED Texts Part III Preparation of Copper(II) Oxide From Copper Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) (i) manganese dioxide and copper (ii). Distinguish between the following pairs of compounds using the reagent given in the bracket. Manganese dioxide and copper (ii) oxide, (using. If no gas is evolved it is cuo. (i) when each of the compound is heated with conc. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.numerade.com
SOLVED Copper (II) oxide reacts with dilute hydrochloric acid. CuO(s Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. (i) when each of the compound is heated with conc. Manganese dioxide and copper (ii) oxide, (using. Study the flow chart and give balanced equations with conditions for the. (i). Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From stock.adobe.com
Vetor de Redox Reaction Infographic Diagram with example of manganese Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Greenish coloured gas is evolved mno 2 + 4hcl. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.ceramic-glazes.com
The Magic of Manganese Dioxide What It Is and Why You Should Care Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) If no gas is evolved it is cuo. Manganese dioxide and copper (ii) oxide, (using. Distinguish between the following pairs of compounds using the reagent given in the bracket. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. Hydrochloric acid, greenish yellow (chlorine) gas is evolved. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Distinguish between the following pairs of compounds using the reagent given in the bracket. Study the flow chart and give balanced equations with conditions for the. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o +. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From link.springer.com
Copper(II) oxide nanocatalyst preparation and characterization green Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Manganese dioxide and copper (ii) oxide, (using. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Study the flow chart and give balanced equations with conditions for the. Distinguish between the following pairs of compounds using the reagent given. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From segudanginformasibagus25.blogspot.com
Uses Of Manganese Iv Oxide In A Dry Cell IT 's Ass Science in your Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Distinguish between the following pairs of compounds using the reagent given in the. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From segudanggambar880.blogspot.com
Manganese Dioxide Balanced Reaction Manganese Dioxide Wikipedia / 1 Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Manganese dioxide and copper (ii) oxide, (using. (i) when each of the compound is heated with conc. Distinguish between the following pairs of compounds using the reagent given in the bracket. Study the flow chart and give balanced equations with conditions for the. If no gas is evolved it is cuo. Only manganese dioxide gives a gaseous product. Hydrochloric acid,. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. (i) manganese dioxide and copper (ii). Study the flow chart and give balanced equations with conditions for the. Distinguish between the following pairs of compounds using the reagent given in. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) when each of the compound is heated with conc. Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. If no gas is evolved it is cuo. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i). Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From recarburizer.en.made-in-china.com
Copper Oxide and Manganese Dioxide Catalyst Catalyst to Convert Carbon Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. (i) when each of the compound is heated with conc. Study the flow chart and give balanced equations with conditions for the. (i) manganese dioxide and copper (ii). Greenish coloured. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From pubs.acs.org
Understanding the Role of Manganese Dioxide in the Oxidation of Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Only manganese dioxide gives a gaseous product. Distinguish between the following pairs of compounds using the reagent given in the bracket. Study the flow chart and give balanced equations with conditions for the. Manganese dioxide and copper (ii) oxide, (using. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Manganese dioxide mno 2 and copper (ii). Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From chemistry.stackexchange.com
chemistry Reaction intermediates of MnO2 catalyzed H2O2 Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: (i) manganese dioxide and copper (ii). Only manganese dioxide gives a gaseous product. Distinguish between the following pairs of compounds using the reagent given in the bracket. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. If no gas. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From brainly.in
On adding dilute hcl to Copper oxide powder the solution formed blue Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Only manganese dioxide gives a gaseous product. Distinguish between the following pairs of compounds using the reagent given in the bracket. Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Manganese dioxide and copper (ii) oxide, (using. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Distinguish between. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Manganese dioxide and copper (ii) oxide, (using. Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Study the flow chart and give balanced equations with conditions for the. Distinguish between the following pairs of compounds using the reagent given in the bracket. If. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.sciencephoto.com
Copper (II) oxide reacts with hydrochloric acid Stock Image C036 Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. Distinguish between the following pairs of compounds using the reagent given in the bracket. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Manganese dioxide mno 2 and copper (ii) oxide cuo can. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From exokdllix.blob.core.windows.net
Lab Preparation Of Co2 Gas at Daniel Champlin blog Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) (i) when each of the compound is heated with conc. If no gas is evolved it is cuo. Manganese dioxide and copper (ii) oxide, (using. Distinguish between the following pairs of compounds using the reagent given in the bracket. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Give one similarity in. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.mdpi.com
Materials Free FullText Fabrication of SinglePhase Manganese Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. If no gas is evolved it is cuo. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Study the flow chart and give balanced equations. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.teachoo.com
Case Base MCQ The reaction between MnO2 with HCl is depicted in Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) If no gas is evolved it is cuo. Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Distinguish between the following pairs of compounds using the reagent given in the bracket. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) manganese dioxide and copper (ii). Only manganese dioxide gives a. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From woelen.homescience.net
Science made alive Chemistry/Solutions Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Distinguish between the following pairs of compounds using the reagent given in the bracket. Study the flow chart and give balanced equations with conditions for the. (i) when each of the compound is heated with conc. (i) manganese dioxide and copper (ii). If no gas is evolved it is cuo. Greenish coloured gas is evolved mno 2 + 4hcl →. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From loeqiuhot.blob.core.windows.net
Electrolysis Of Copper (Ii) Chloride at Faviola Thompson blog Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) (i) manganese dioxide and copper (ii). Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Distinguish between the following pairs of compounds using the reagent given in the bracket. Distinguish between the following pairs of compounds using the reagent. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From exotsdtks.blob.core.windows.net
Copper (Ii) Oxide Formula at Travis Summers blog Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Manganese dioxide and copper (ii) oxide, (using. Only manganese dioxide gives a gaseous product. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. If no gas is evolved it is cuo. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.scienceabc.com
Manganese Oxide Chemical Formula, Properties And Uses » ScienceABC Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) (i) manganese dioxide and copper (ii). (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Distinguish between the following pairs of compounds using the reagent given in the bracket. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. If no gas is evolved it is cuo. Greenish coloured. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From polizneuro.weebly.com
Reactivity series of metals polizneuro Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Distinguish between the following pairs of compounds using the reagent given in the bracket. Manganese dioxide and copper (ii) oxide, (using. Distinguish between the following pairs of compounds using the reagent given in the bracket. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Distinguish between the following pairs of compounds using the reagent given in the bracket. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in. Manganese dioxide and copper (ii) oxide,. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.chegg.com
Solved Correct PartC olid copper (II) oxide reacts with Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) If no gas is evolved it is cuo. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: (i) manganese dioxide and copper (ii). Only manganese dioxide gives a gaseous product. Manganese dioxide and copper (ii) oxide, (using. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.numerade.com
SOLVEDA common demonstration in chemistry courses involves adding a Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Only manganese dioxide gives a gaseous product. Manganese dioxide and copper (ii) oxide, (using. (i) when each of the compound is heated with conc. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Greenish coloured gas is evolved mno 2 + 4hcl → mncl 2 + 2h 2 o + cl 2 filterate is brownish in.. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Distinguish between the following pairs of compounds using the reagent given in the bracket. If no. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) If no gas is evolved it is cuo. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Manganese dioxide and copper (ii) oxide, (using. (i) on adding concentrated hydrochloric acid if a greenish yellow gas. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Distinguish between the following pairs of compounds using the reagent given in the bracket. Manganese dioxide mno 2 and copper (ii) oxide cuo can be distinguished by using the concentrated hcl: Assertion :both copper oxide and manganese dioxide gives reaction with h c l. (i) when each of the compound is heated with conc. Only manganese dioxide gives a gaseous. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From loeqyugeg.blob.core.windows.net
Copper(Ii) Oxide And Carbon Equation at John Wyllie blog Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) If no gas is evolved it is cuo. Assertion :both copper oxide and manganese dioxide gives reaction with h c l. Distinguish between the following pairs of compounds using the reagent given in the bracket. Give one similarity in the test between (i)cl 2 and hcl (ii) so 2 and co 2. Manganese dioxide mno 2 and copper (ii) oxide. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).
From www.pinterest.co.uk
Manganese compounds colors (different oxidation states) chemistry Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl) Hydrochloric acid, greenish yellow (chlorine) gas is evolved in case of manganese dioxide. Distinguish between the following pairs of compounds using the reagent given in the bracket. (i) on adding concentrated hydrochloric acid if a greenish yellow gas is evolved it is manganese dioxide. Manganese dioxide and copper (ii) oxide, (using. Study the flow chart and give balanced equations with. Manganese Dioxide And Copper (Ii) Oxide. (Using Concentrated Hcl).