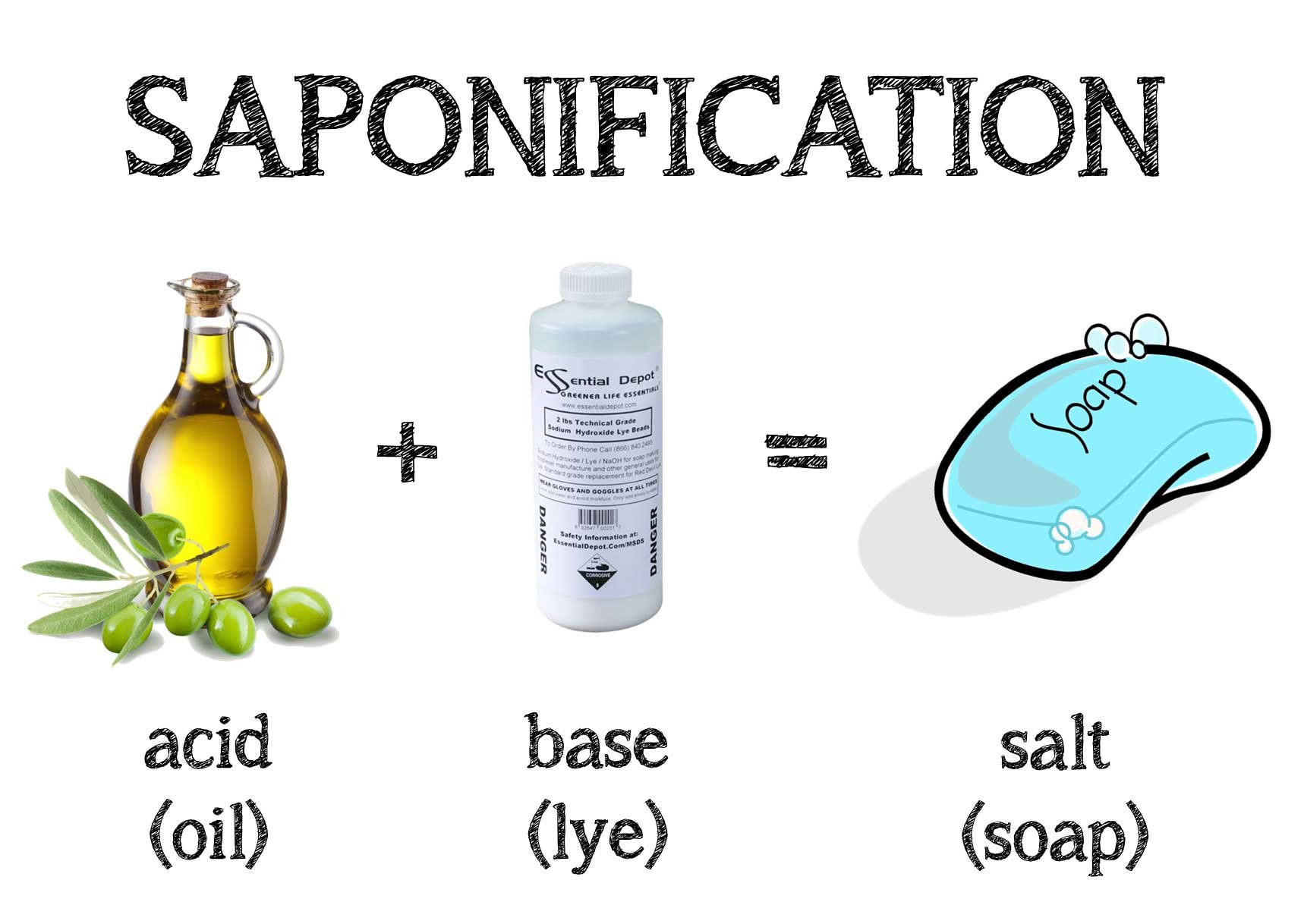
Soap Making Chemical Reaction . Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. When the fat and water no longer separated, the mixture was. Ester + base (sodium/ potassium hydroxide) → alcohol + soap the ester reacts. Saponification is a broad term used to describe the chemical reaction involved in the production of soap. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that convert raw. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is the name of the chemical reaction that produces soap. The chemical structures of fats and. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. Here is the fundamental chemical reaction: Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph).
from busy.org
When the fat and water no longer separated, the mixture was. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. Saponification is the name of the chemical reaction that produces soap. The chemical structures of fats and. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. Ester + base (sodium/ potassium hydroxide) → alcohol + soap the ester reacts. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol.
Saponification 1/2
Soap Making Chemical Reaction In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. Saponification is a broad term used to describe the chemical reaction involved in the production of soap. Here is the fundamental chemical reaction: One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Saponification is the name of the chemical reaction that produces soap. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Ester + base (sodium/ potassium hydroxide) → alcohol + soap the ester reacts. The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that convert raw. The chemical structures of fats and. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. When the fat and water no longer separated, the mixture was.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soap Making Chemical Reaction Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. Saponification is a broad term used to describe the chemical reaction involved in the production of soap. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Ester + base (sodium/ potassium hydroxide) → alcohol + soap. Soap Making Chemical Reaction.
From in.pinterest.com
Hand washing with soap vector illustration. Educational explanation Soap Making Chemical Reaction Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and. Soap Making Chemical Reaction.
From www.stephensonpersonalcare.com
Ingredient Spotlight Oils Used in the Soap Making Process Stephenson Soap Making Chemical Reaction Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. The chemistry behind handmade. Soap Making Chemical Reaction.
From www.scribd.com
The Chemical Reaction of Soap Making Chemistry Physical Sciences Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. In the process, animal or vegetable fat. Soap Making Chemical Reaction.
From ar.inspiredpencil.com
Preparation Of Soap In Chemistry Project Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. The chemistry behind handmade soap making is fascinating,. Soap Making Chemical Reaction.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Soap Soap Making Chemical Reaction The chemical structures of fats and. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Here is the fundamental chemical reaction: The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. The chemistry behind handmade soap making is fascinating, involving a series of reactions. Soap Making Chemical Reaction.
From www.slideserve.com
PPT Soap Describe how soap is made from fatty acids and alkalis Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. When the fat and water no longer separated, the mixture was. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. The chemical structures of fats and. The chemistry behind handmade soap making is fascinating, involving a series of. Soap Making Chemical Reaction.
From historymeetsscience.blogspot.com
Tales of scientific journeys Soap making 101 Soap Making Chemical Reaction The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is the name of the chemical reaction that produces soap. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Saponification is a broad. Soap Making Chemical Reaction.
From www.youtube.com
Hydrolysis & How It Is Used To Make Soaps Organic Chemistry Soap Making Chemical Reaction The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that convert raw. When the fat and water no longer separated, the mixture was. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Natural soaps are sodium or potassium salts of fatty acids, originally made. Soap Making Chemical Reaction.
From spmchemistry.blog.onlinetuition.com.my
The Making of Detergent SPM Chemistry Soap Making Chemical Reaction Saponification is a broad term used to describe the chemical reaction involved in the production of soap. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Soap is made from reacting a. Soap Making Chemical Reaction.
From thesoapmoleculeco.com
The Soap Molecule Co. Soap Making Chemical Reaction The chemical structures of fats and. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that convert raw. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water. Soap Making Chemical Reaction.
From www.thoughtco.com
Saponification Definition and Reaction Soap Making Chemical Reaction The chemical structures of fats and. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that. Soap Making Chemical Reaction.
From www.youtube.com
[5.1] Preparation of soap YouTube Soap Making Chemical Reaction The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. The chemical structures of fats and. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in. Soap Making Chemical Reaction.
From labmuffin.com
Make Your Own Soap! Part 1 The Chemistry Behind Soap Making Lab Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is a broad term used to describe the chemical reaction involved in the production of soap. Slowly, a chemical reaction called saponification would take place between the fat and. Soap Making Chemical Reaction.
From study.com
Saponification Definition, Reaction & Mechanism Lesson Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that convert raw. The chemical structures of fats and. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Soaps consist of extended molecular structures. Soap Making Chemical Reaction.
From stock.adobe.com
Saponification equation, reaction of soap, chemistry equation of soap Soap Making Chemical Reaction The chemical structures of fats and. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is a broad term used to describe the chemical reaction involved in the production of soap. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Saponification is. Soap Making Chemical Reaction.
From www.sharpestarena.com
Production of Soap Complete Project on Soap Making Soap Making Chemical Reaction Saponification is a broad term used to describe the chemical reaction involved in the production of soap. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Here is the fundamental chemical reaction: Slowly, a chemical reaction called saponification would take place between the. Soap Making Chemical Reaction.
From learnphysics-dhruv.blogspot.com
Physics Learn To prepare Soap by cold process. Science practical GSEB Soap Making Chemical Reaction Here is the fundamental chemical reaction: One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with. Soap Making Chemical Reaction.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soap Making Chemical Reaction The chemical structures of fats and. Saponification is the name of the chemical reaction that produces soap. Ester + base (sodium/ potassium hydroxide) → alcohol + soap the ester reacts. Here is the fundamental chemical reaction: One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Slowly, a chemical reaction. Soap Making Chemical Reaction.
From www.lolaapp.com
The Chemistry of Soap Unveiling the Reactions Behind Production Soap Making Chemical Reaction One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Saponification is the name of the chemical reaction that produces soap. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Saponification is a broad term used to describe. Soap Making Chemical Reaction.
From joitjxuuu.blob.core.windows.net
Making Soap In Chemistry Class at Willis Jones blog Soap Making Chemical Reaction Ester + base (sodium/ potassium hydroxide) → alcohol + soap the ester reacts. The chemical structures of fats and. Here is the fundamental chemical reaction: The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is a broad term used to describe the chemical reaction involved in the production of. Soap Making Chemical Reaction.
From www.thoughtco.com
How Saponification Makes Soap Soap Making Chemical Reaction Ester + base (sodium/ potassium hydroxide) → alcohol + soap the ester reacts. One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. The chemical structures of fats and. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. In the. Soap Making Chemical Reaction.
From www.vedantu.com
Calcium hydroxide is used for making soapA. TrueB. False Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. When the fat and water no longer separated, the mixture was. The chemical structures of fats and. The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that convert raw. One of the organic chemical reactions known to ancient man was the preparation of. Soap Making Chemical Reaction.
From studylib.net
Chemical Reactions Soap Making Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. Saponification is a broad term used to describe the chemical reaction involved in the production of soap. The chemical structures of fats and. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide).. Soap Making Chemical Reaction.
From www.youtube.com
Cleansing action of soap Chemical reactions Chemistry YouTube Soap Making Chemical Reaction One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Slowly, a chemical reaction called saponification would take place between the fat and the hydroxide which resulted in a liquid soap. When the fat and water no longer separated, the mixture was. The chemical structures of fats and. In the. Soap Making Chemical Reaction.
From www.kremica.si
Kako nastane milo ali Vse o saponifikaciji • Kremica Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). Saponification is a broad term used to describe the chemical reaction involved in the production of soap. Slowly, a chemical reaction called saponification would take place between the. Soap Making Chemical Reaction.
From www.youtube.com
Soap Making Process YouTube Soap Making Chemical Reaction Here is the fundamental chemical reaction: Saponification is a broad term used to describe the chemical reaction involved in the production of soap. Saponification is the name of the chemical reaction that produces soap. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. The chemistry behind handmade soap making is. Soap Making Chemical Reaction.
From thenerdyfarmwife.com
Why do you need lye to make soap? (FAQS) Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. One of the organic chemical reactions known to ancient man was the preparation. Soap Making Chemical Reaction.
From www.pinterest.com
An Introduction to Chemistry Soap, Soap making, Chemical reactions Soap Making Chemical Reaction One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Saponification is the name of the chemical reaction that produces soap. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). Here is the. Soap Making Chemical Reaction.
From busy.org
Saponification 1/2 Soap Making Chemical Reaction Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). The chemical structures of fats and. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water. Soap Making Chemical Reaction.
From www.shutterstock.com
Saponification Equation Reaction Soap Chemistry Equation Stock Soap Making Chemical Reaction The chemical structures of fats and. Soaps consist of extended molecular structures composed of sodium (na) and potassium (k) salts of fatty acids. When the fat and water no longer separated, the mixture was. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is the name of the chemical. Soap Making Chemical Reaction.
From www.saubhaya.com
Chemical Makeup Of Soap Saubhaya Makeup Soap Making Chemical Reaction Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). The chemical structures of fats and. The reaction requires a solution of an alkali (e.g., sodium hydroxide or potassium hydroxide) in water and also heat. Saponification is a broad term used to describe the. Soap Making Chemical Reaction.
From www.slideshare.net
Chemistry of soaps Soap Making Chemical Reaction In the process, animal or vegetable fat is converted into soap (a fatty acid) and alcohol. Soap is made from reacting a fat or oil (or a mixture) with a strong base (something with very high ph). When the fat and water no longer separated, the mixture was. The chemical structures of fats and. Saponification is the name of the. Soap Making Chemical Reaction.
From www.alamy.com
Soap Preparation Atomic representation of Saponification reaction Soap Making Chemical Reaction Ester + base (sodium/ potassium hydroxide) → alcohol + soap the ester reacts. Saponification is the name of the chemical reaction that produces soap. Natural soaps are sodium or potassium salts of fatty acids, originally made by boiling lard or other animal fat together with lye or potash (potassium hydroxide). The reaction requires a solution of an alkali (e.g., sodium. Soap Making Chemical Reaction.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 Soap Making Chemical Reaction Saponification is the name of the chemical reaction that produces soap. The chemistry behind handmade soap making is fascinating, involving a series of reactions and processes that convert raw. Here is the fundamental chemical reaction: One of the organic chemical reactions known to ancient man was the preparation of soaps through a reaction called saponification. Ester + base (sodium/ potassium. Soap Making Chemical Reaction.