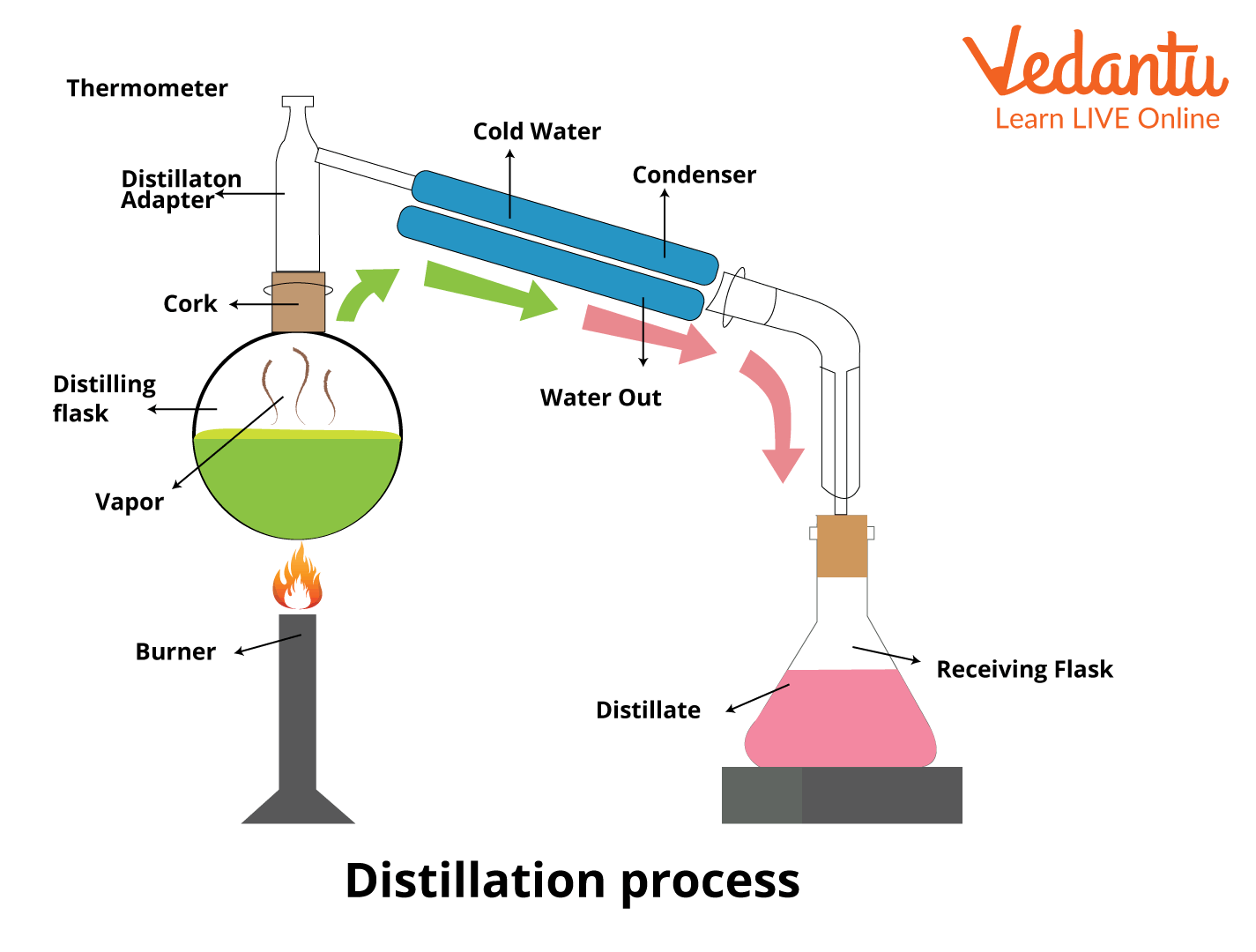
Purpose Of A Distillation . Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. We utilize the difference in boiling points of liquids as a basis of separation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. It is a separation technique that can be used to either increase the concentration of a. An appropriate distillation collects distillate at a rate of 1 drop per second. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation.
from www.vedantu.com
It is a separation technique that can be used to either increase the concentration of a. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. An appropriate distillation collects distillate at a rate of 1 drop per second. We utilize the difference in boiling points of liquids as a basis of separation.
Uses of Distillation Learn Important Terms and Concepts
Purpose Of A Distillation It is a separation technique that can be used to either increase the concentration of a. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. An appropriate distillation collects distillate at a rate of 1 drop per second. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. We utilize the difference in boiling points of liquids as a basis of separation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification technique for a liquid or a mixture of liquids. It is a separation technique that can be used to either increase the concentration of a.
From www.geeksforgeeks.org
Distillation Definition, Meaning, Principle, Types & Uses Purpose Of A Distillation Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. An appropriate distillation collects distillate at a rate of 1 drop per second. Distillation is a purification technique for a liquid or a mixture of liquids. We utilize the difference in boiling points of liquids as. Purpose Of A Distillation.
From www.shalom-education.com
Simple Distillation GCSE Chemistry Revision Purpose Of A Distillation It is a separation technique that can be used to either increase the concentration of a. Distillation is a purification technique for a liquid or a mixture of liquids. An appropriate distillation collects distillate at a rate of 1 drop per second. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor. Purpose Of A Distillation.
From chemicaltweak.com
6 Types Of Distillation And Definition [Explained In Detail] Purpose Of A Distillation It is a separation technique that can be used to either increase the concentration of a. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. We. Purpose Of A Distillation.
From fyomibolg.blob.core.windows.net
How Does A Distillation Apparatus Work at Mildred Wilkerson blog Purpose Of A Distillation We utilize the difference in boiling points of liquids as a basis of separation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is a purification method for liquids, and can separate components of a mixture if they have. Purpose Of A Distillation.
From psiberg.com
Fractional Distillation Uses, Working, and Apparatus PSIBERG Purpose Of A Distillation Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. An appropriate distillation collects distillate at a rate of 1 drop per second. We utilize the difference in boiling points. Purpose Of A Distillation.
From www.thoughtco.com
What Is Distillation? Principles and Uses Purpose Of A Distillation We utilize the difference in boiling points of liquids as a basis of separation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. It is a separation technique that can be used to either increase the concentration of a. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases,. Purpose Of A Distillation.
From www.vedantu.com
Uses of Distillation Learn Important Terms and Concepts Purpose Of A Distillation Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. We utilize the difference in boiling points of liquids as a basis of separation. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. An appropriate distillation collects distillate at. Purpose Of A Distillation.
From easywayscience78.blogspot.com
Distillation Easy way to learn science Purpose Of A Distillation Distillation is a purification technique for a liquid or a mixture of liquids. We utilize the difference in boiling points of liquids as a basis of separation. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. Distillation is used to separate liquids from nonvolatile solids, as in the separation of. Purpose Of A Distillation.
From www.chemicalslearning.com
Simple Distillation Process Differential Distillation Process Purpose Of A Distillation Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. It is a separation technique that can be used to either increase the concentration of a. An appropriate distillation collects distillate at a. Purpose Of A Distillation.
From chemistnotes.com
Distillation Definition and Types of Distillation Chemistry Notes Purpose Of A Distillation We utilize the difference in boiling points of liquids as a basis of separation. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification technique for a liquid or a mixture. Purpose Of A Distillation.
From www.youtube.com
Distillation Definition and Technique Basic Principles and Purpose Of A Distillation Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distillation is a purification technique for a liquid or a mixture of liquids. It is a separation. Purpose Of A Distillation.
From neutrium.net
Distillation Fundamentals Neutrium Purpose Of A Distillation Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. We utilize the difference in boiling points of liquids as a basis of separation. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. Distillation is a purification technique for. Purpose Of A Distillation.
From www.peoplesbourbonreview.com
What is Distillation and How is Liquor Made? The People's Bourbon Review Purpose Of A Distillation Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. It is a separation technique that can be used to either increase the concentration of a. An appropriate distillation collects distillate at a rate of 1 drop per second. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and. Purpose Of A Distillation.
From www.wermac.org
Distillation Column Basic Distillation Equipment and Operation Purpose Of A Distillation Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. An appropriate distillation collects distillate at a rate of 1 drop per second. It is a separation technique that can be used to. Purpose Of A Distillation.
From cekkmchw.blob.core.windows.net
Purpose Of A Distillation Unit at Elizabeth Powell blog Purpose Of A Distillation Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. An appropriate distillation collects distillate at a rate of 1 drop per second. Distillation is a purification technique for a liquid or a mixture of liquids. We utilize the difference in boiling points of liquids as a basis of. Purpose Of A Distillation.
From testbook.com
team Distillation Principle, Working, Advantages & Application Purpose Of A Distillation We utilize the difference in boiling points of liquids as a basis of separation. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. It is a separation technique that can be used to either. Purpose Of A Distillation.
From www.expii.com
Separating Mixtures — Overview & Common Methods Expii Purpose Of A Distillation Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. We utilize the difference in boiling points of liquids as a basis of separation. Distillation is used to separate liquids from nonvolatile solids,. Purpose Of A Distillation.
From cekkmchw.blob.core.windows.net
Purpose Of A Distillation Unit at Elizabeth Powell blog Purpose Of A Distillation Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. We utilize the difference in boiling points of liquids as a basis of separation. An appropriate distillation collects distillate at. Purpose Of A Distillation.
From glossary.periodni.com
Distillation Chemistry Dictionary & Glossary Purpose Of A Distillation Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. We utilize the difference in boiling points of liquids as a basis of separation. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. Distillation is a. Purpose Of A Distillation.
From www.geeksforgeeks.org
Separation by Distillation Purpose Of A Distillation Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. It is a separation technique that can be used to either increase the concentration of a. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification method for liquids, and can. Purpose Of A Distillation.
From keystagewiki.com
Distillation Key Stage Wiki Purpose Of A Distillation Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. It is a separation technique that can be used to either increase the concentration of. Purpose Of A Distillation.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts Purpose Of A Distillation Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. Distillation is a purification technique for a liquid or a mixture of liquids. We utilize the difference in boiling points of liquids as a basis of separation. Distillation refers to the selective boiling and subsequent condensation of a component. Purpose Of A Distillation.
From www.theengineersperspectives.com
How Does Simple Distillation Work? The Engineer's Perspective Purpose Of A Distillation Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. We utilize the difference in boiling points of liquids as a basis of separation. Distillation is a purification technique for a liquid or. Purpose Of A Distillation.
From speichim.com
Main principes of distillation Speichim Processing Valls Química Purpose Of A Distillation Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. An appropriate distillation collects distillate at a rate of 1 drop per second. We utilize the difference. Purpose Of A Distillation.
From www.yaclass.in
Simple distillation — lesson. Science CBSE, Class 9. Purpose Of A Distillation Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. It is a separation technique that can be used to either increase the concentration of a. Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is used to separate liquids from nonvolatile solids, as. Purpose Of A Distillation.
From www.chemicals.co.uk
Distillation Of A Product From A Reaction The Chemistry Blog Purpose Of A Distillation Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. We utilize the difference in boiling points of liquids as a basis of separation. It is a separation technique that can be used to either increase the concentration of a. Distillation refers to the selective boiling and subsequent condensation. Purpose Of A Distillation.
From www.youtube.com
What is Simple Distillation Separation Methods Chemistry Basics YouTube Purpose Of A Distillation We utilize the difference in boiling points of liquids as a basis of separation. Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. It is a separation technique that can be used to either increase the concentration of a. Distillation is used to separate liquids from nonvolatile solids,. Purpose Of A Distillation.
From www.teachoo.com
What are the Properties of Mixtures? Teachoo Concepts Purpose Of A Distillation Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. An appropriate distillation collects distillate at a rate of 1 drop per second. We utilize the difference in boiling points of liquids as a basis of separation. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in. Purpose Of A Distillation.
From www.theengineeringconcepts.com
Types of Distillation The Engineering Concepts Purpose Of A Distillation An appropriate distillation collects distillate at a rate of 1 drop per second. We utilize the difference in boiling points of liquids as a basis of separation. Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in. Purpose Of A Distillation.
From www.alamy.com
Distillation process diagram for education illustration Stock Vector Purpose Of A Distillation Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. It is a separation technique that can be used to either increase the concentration of a. An appropriate distillation collects distillate at a. Purpose Of A Distillation.
From www.chemicals.co.uk
What is the Distillation Process? The Chemistry Blog Purpose Of A Distillation We utilize the difference in boiling points of liquids as a basis of separation. An appropriate distillation collects distillate at a rate of 1 drop per second. Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or. Purpose Of A Distillation.
From de.wikipedia.org
Destillation Wikipedia Purpose Of A Distillation Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. It is a separation technique that can be used to either increase the concentration of a. An. Purpose Of A Distillation.
From www.slideserve.com
PPT Separation Techniques Grade 10 Chemistry PowerPoint Presentation Purpose Of A Distillation Distillation refers to the selective boiling and subsequent condensation of a component in a liquid mixture. Distilling at too fast a rate prevents proper equilibration between liquid and gas phases, and results in poor separation. It is a separation technique that can be used to either increase the concentration of a. We utilize the difference in boiling points of liquids. Purpose Of A Distillation.
From www.youtube.com
how to prepare distilled water distilled water preparation in Purpose Of A Distillation Distillation is a purification method for liquids, and can separate components of a mixture if they have significantly different boiling points. We utilize the difference in boiling points of liquids as a basis of separation. Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. Distillation. Purpose Of A Distillation.
From zamcopter.web.fc2.com
SIMPLE DISTILLATION Purpose Of A Distillation Distillation is used to separate liquids from nonvolatile solids, as in the separation of alcoholic liquors from fermented materials, or in the separation of. We utilize the difference in boiling points of liquids as a basis of separation. Distillation is a purification technique for a liquid or a mixture of liquids. Distillation is a purification method for liquids, and can. Purpose Of A Distillation.