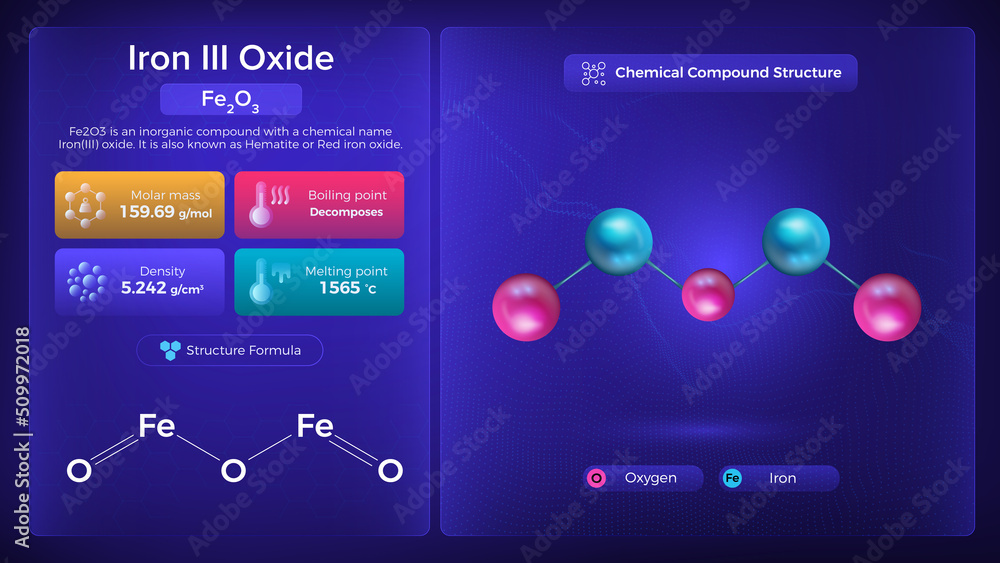
How To Write Iron Iii Oxide . When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. In this video we'll write the correct formula for iron (iii) oxide. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). How to write the formula for iron (iii) oxide. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. To write the formula for iron (iii) oxide we’ll use the periodic table, a. The chemical equation for this reaction is: Moreover, the main ones are α and γ, iron adopts octahedral coordination. The formula is derived by. The formula of iron (iii) oxide is given as fe203. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Its chemical formula is fe2o3. The oxidation state of fe 2 o 3 is +3.
from stock.adobe.com
It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. To write the formula for iron (iii) oxide we’ll use the periodic table, a. The chemical equation for this reaction is: The formula is derived by. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. The oxidation state of fe 2 o 3 is +3. Its chemical formula is fe2o3. In this video we'll write the correct formula for iron (iii) oxide.
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector
How To Write Iron Iii Oxide To write the formula for iron (iii) oxide we’ll use the periodic table, a. The chemical equation for this reaction is: Moreover, the main ones are α and γ, iron adopts octahedral coordination. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. The formula of iron (iii) oxide is given as fe203. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Its chemical formula is fe2o3. For iron (iii) oxide use the hints and resources below to help write the formula. The formula is derived by. To write the formula for iron (iii) oxide we’ll use the periodic table, a. The oxidation state of fe 2 o 3 is +3. In this video we'll write the correct formula for iron (iii) oxide. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry.
From www.slideserve.com
PPT Naming Type (I) and (II) Compounds PowerPoint Presentation, free How To Write Iron Iii Oxide 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). How to write the formula for iron (iii) oxide. The formula is derived. How To Write Iron Iii Oxide.
From www.thoughtco.com
Easy Steps for Balancing Chemical Equations How To Write Iron Iii Oxide Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Moreover, the main ones are α and γ, iron adopts octahedral coordination. For iron (iii) oxide use the hints and resources below to help write. How To Write Iron Iii Oxide.
From www.slideserve.com
PPT Combustion analysis PowerPoint Presentation, free download ID How To Write Iron Iii Oxide In this video we'll write the correct formula for iron (iii) oxide. To write the formula for iron (iii) oxide we’ll use the periodic table, a. The formula is derived by. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. The formula of iron (iii) oxide is given as fe203. Its molar mass is 159.69 g/mol and we can obtain it. How To Write Iron Iii Oxide.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses How To Write Iron Iii Oxide Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. To write the formula for iron (iii) oxide we’ll use the periodic table, a. The formula is derived by. Moreover, the main ones are α and γ, iron adopts octahedral coordination. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Its chemical formula is fe2o3. Both methods. How To Write Iron Iii Oxide.
From www.slideserve.com
PPT Nomenclature PowerPoint Presentation, free download How To Write Iron Iii Oxide To write the formula for iron (iii) oxide we’ll use the periodic table, a. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. For iron (iii) oxide use the hints and resources below to help write the formula. 2𝐹𝑒 (𝑂𝐻)₃. How To Write Iron Iii Oxide.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to How To Write Iron Iii Oxide When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. For iron (iii) oxide. How To Write Iron Iii Oxide.
From slideplayer.com
Ionic Compounds Naming ppt download How To Write Iron Iii Oxide The oxidation state of fe 2 o 3 is +3. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. To write the formula for iron (iii) oxide we’ll use the periodic table, a. How to write the formula for iron (iii) oxide. It is also referred to as ferric oxide, red iron oxide, iron trioxide,. How To Write Iron Iii Oxide.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate How To Write Iron Iii Oxide Its chemical formula is fe2o3. Moreover, the main ones are α and γ, iron adopts octahedral coordination. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The oxidation state of fe 2 o. How To Write Iron Iii Oxide.
From www.youtube.com
Iron(III) oxide Meaning YouTube How To Write Iron Iii Oxide Moreover, the main ones are α and γ, iron adopts octahedral coordination. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. To write the formula for iron (iii) oxide we’ll use the periodic table, a. In this video we'll write the correct formula for iron (iii) oxide.. How To Write Iron Iii Oxide.
From www.youtube.com
Al+Fe2O3=Al2O3+Fe Balanced EquationAluminum+Iron(III) oxide+Iron How To Write Iron Iii Oxide How to write the formula for iron (iii) oxide. The chemical equation for this reaction is: The formula is derived by. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Its chemical formula is fe2o3. To write the formula for iron (iii) oxide we’ll use the periodic table, a. In this video we'll. How To Write Iron Iii Oxide.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd How To Write Iron Iii Oxide Its chemical formula is fe2o3. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. For iron (iii) oxide use the hints and resources below to help write the formula. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel. How To Write Iron Iii Oxide.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube How To Write Iron Iii Oxide The formula is derived by. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The chemical equation for this. How To Write Iron Iii Oxide.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry How To Write Iron Iii Oxide How to write the formula for iron (iii) oxide. The formula of iron (iii) oxide is given as fe203. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Moreover, the main ones are α and γ, iron adopts octahedral coordination. It is also referred to as ferric oxide, red iron oxide, iron trioxide,. How To Write Iron Iii Oxide.
From www.youtube.com
Naming Ionic compounds Iron (III) Oxide YouTube How To Write Iron Iii Oxide The chemical equation for this reaction is: How to write the formula for iron (iii) oxide. Moreover, the main ones are α and γ, iron adopts octahedral coordination. The formula of iron (iii) oxide is given as fe203. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy). How To Write Iron Iii Oxide.
From www.slideserve.com
PPT iBar A Solution To Ionic Naming PowerPoint Presentation, free How To Write Iron Iii Oxide The formula is derived by. How to write the formula for iron (iii) oxide. The oxidation state of fe 2 o 3 is +3. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). Its chemical formula is fe2o3. When you heat iron (iii) hydroxide, it breaks down to form iron. How To Write Iron Iii Oxide.
From webgiasi.vn
How to write the equation for Fe2O3 + H2O Iron (III) oxide + Water How To Write Iron Iii Oxide The chemical equation for this reaction is: Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The formula of iron (iii) oxide is given as fe203. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. Iron(iii) oxide. How To Write Iron Iii Oxide.
From stock.adobe.com
Iron Iii Oxide Properties and Chemical Compound Structure Stock Vector How To Write Iron Iii Oxide The oxidation state of fe 2 o 3 is +3. How to write the formula for iron (iii) oxide. In this video we'll write the correct formula for iron (iii) oxide. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). For iron (iii) oxide use the hints and resources below. How To Write Iron Iii Oxide.
From testbook.com
Oxide Learn Structure, Preparation, Properties & Uses How To Write Iron Iii Oxide 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). The chemical equation for this reaction is: How to write the formula for iron (iii) oxide. In this video we'll write the correct formula for iron (iii) oxide. Both methods result in the formation of. How To Write Iron Iii Oxide.
From slidetodoc.com
Writing and Naming Chemical Formulas for Ionic Compounds How To Write Iron Iii Oxide When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. For iron (iii) oxide use the hints and resources below to help write the formula. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. How to write. How To Write Iron Iii Oxide.
From www.numerade.com
SOLVEDThe rusting of iron is a chemical reaction of iron with oxygen How To Write Iron Iii Oxide Moreover, the main ones are α and γ, iron adopts octahedral coordination. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. In this video we'll write the correct formula for iron (iii) oxide. Its. How To Write Iron Iii Oxide.
From en.wikipedia.org
Iron(III) oxide Wikipedia How To Write Iron Iii Oxide Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. For iron (iii) oxide use the hints and resources below to help write the formula. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). Moreover, the main ones are. How To Write Iron Iii Oxide.
From stock.adobe.com
iron III Oxide Properties and Chemical Compound Structure Stock Vector How To Write Iron Iii Oxide The formula of iron (iii) oxide is given as fe203. In this video we'll write the correct formula for iron (iii) oxide. Moreover, the main ones are α and γ, iron adopts octahedral coordination. The oxidation state of fe 2 o 3 is +3. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. For iron. How To Write Iron Iii Oxide.
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 How To Write Iron Iii Oxide For iron (iii) oxide use the hints and resources below to help write the formula. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Moreover, the main ones are α and γ, iron adopts octahedral coordination. In this video we'll write the correct formula for iron (iii) oxide. Its chemical formula is fe2o3.. How To Write Iron Iii Oxide.
From www.solutionspile.com
[Solved] Under certain conditions, the substances iron and How To Write Iron Iii Oxide The formula is derived by. The chemical equation for this reaction is: 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. For iron (iii) oxide use the hints and resources below to help write the formula. Moreover, the main ones are α and γ, iron adopts octahedral coordination. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. Its chemical formula is fe2o3. Fe 2. How To Write Iron Iii Oxide.
From testbook.com
Iron(III) Oxide Formula Know Its Preparation, Properties & Uses How To Write Iron Iii Oxide For iron (iii) oxide use the hints and resources below to help write the formula. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. The formula of iron (iii) oxide is given as fe203. The oxidation state of fe 2 o 3 is +3. How to write. How To Write Iron Iii Oxide.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library How To Write Iron Iii Oxide How to write the formula for iron (iii) oxide. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. For iron (iii) oxide use the hints and resources below to help write the formula. To write the formula for iron (iii) oxide we’ll use the periodic table, a. Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. The chemical. How To Write Iron Iii Oxide.
From www.numerade.com
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a How To Write Iron Iii Oxide Both methods result in the formation of iron (iii) oxide, commonly used in various applications, including pigments and the steel industry. The oxidation state of fe 2 o 3 is +3. The chemical equation for this reaction is: For iron (iii) oxide use the hints and resources below to help write the formula. It is also referred to as ferric. How To Write Iron Iii Oxide.
From www.youtube.com
How to Write the Formula for Iron (III) acetate YouTube How To Write Iron Iii Oxide It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. The chemical equation for this reaction is: The formula is derived by. Its molar mass is 159.69 g/mol and we can obtain it by various. How To Write Iron Iii Oxide.
From www.numerade.com
SOLVEDThe formation of rust [Iron (III) oxide] on the surface of iron How To Write Iron Iii Oxide Its chemical formula is fe2o3. For iron (iii) oxide use the hints and resources below to help write the formula. To write the formula for iron (iii) oxide we’ll use the periodic table, a. How to write the formula for iron (iii) oxide. In this video we'll write the correct formula for iron (iii) oxide. The chemical equation for this. How To Write Iron Iii Oxide.
From www.chegg.com
Solved The balanced chemical equation for iron rusting to How To Write Iron Iii Oxide The formula is derived by. Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. The chemical equation for this reaction is: The formula of iron (iii) oxide is given as fe203. Its chemical formula is fe2o3. For iron (iii) oxide use the hints and resources below to help write the formula. Fe 2 o 3 is the chemical formula of iron(iii). How To Write Iron Iii Oxide.
From www.showme.com
Iron III oxide Science, Chemistry, Chemical Bonds ShowMe How To Write Iron Iii Oxide When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. How to write the formula for iron (iii) oxide. The oxidation state of fe 2 o 3 is +3. For iron (iii) oxide use. How To Write Iron Iii Oxide.
From slidetodoc.com
Writing and Naming Chemical Formulas for Ionic Compounds How To Write Iron Iii Oxide In this video we'll write the correct formula for iron (iii) oxide. The formula is derived by. Moreover, the main ones are α and γ, iron adopts octahedral coordination. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). For iron (iii) oxide use the hints and resources below to help. How To Write Iron Iii Oxide.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube How To Write Iron Iii Oxide Iron(iii) oxide has the chemical formula {eq}fe_{2}o_{3} {/eq}. For iron (iii) oxide use the hints and resources below to help write the formula. The oxidation state of fe 2 o 3 is +3. It is also referred to as ferric oxide, red iron oxide, iron trioxide, and oxo (oxoferriooxy) iron (iupac name). The formula is derived by. The formula of. How To Write Iron Iii Oxide.
From www.slideserve.com
PPT Chemical Equations PowerPoint Presentation, free download ID How To Write Iron Iii Oxide To write the formula for iron (iii) oxide we’ll use the periodic table, a. The oxidation state of fe 2 o 3 is +3. Fe 2 o 3 is the chemical formula of iron(iii) oxide which has three oxygen atoms, and two iron atoms. The formula is derived by. The chemical equation for this reaction is: Its molar mass is. How To Write Iron Iii Oxide.
From www.chegg.com
Solved 3. Iron reacts with oxygen to form iron (III) oxide How To Write Iron Iii Oxide Its molar mass is 159.69 g/mol and we can obtain it by various polymorphs. Moreover, the main ones are α and γ, iron adopts octahedral coordination. How to write the formula for iron (iii) oxide. When you heat iron (iii) hydroxide, it breaks down to form iron (iii) oxide and water. 2𝐹𝑒 (𝑂𝐻)₃ → fe₂o₃ + 3𝐻₂𝑂. It is also. How To Write Iron Iii Oxide.