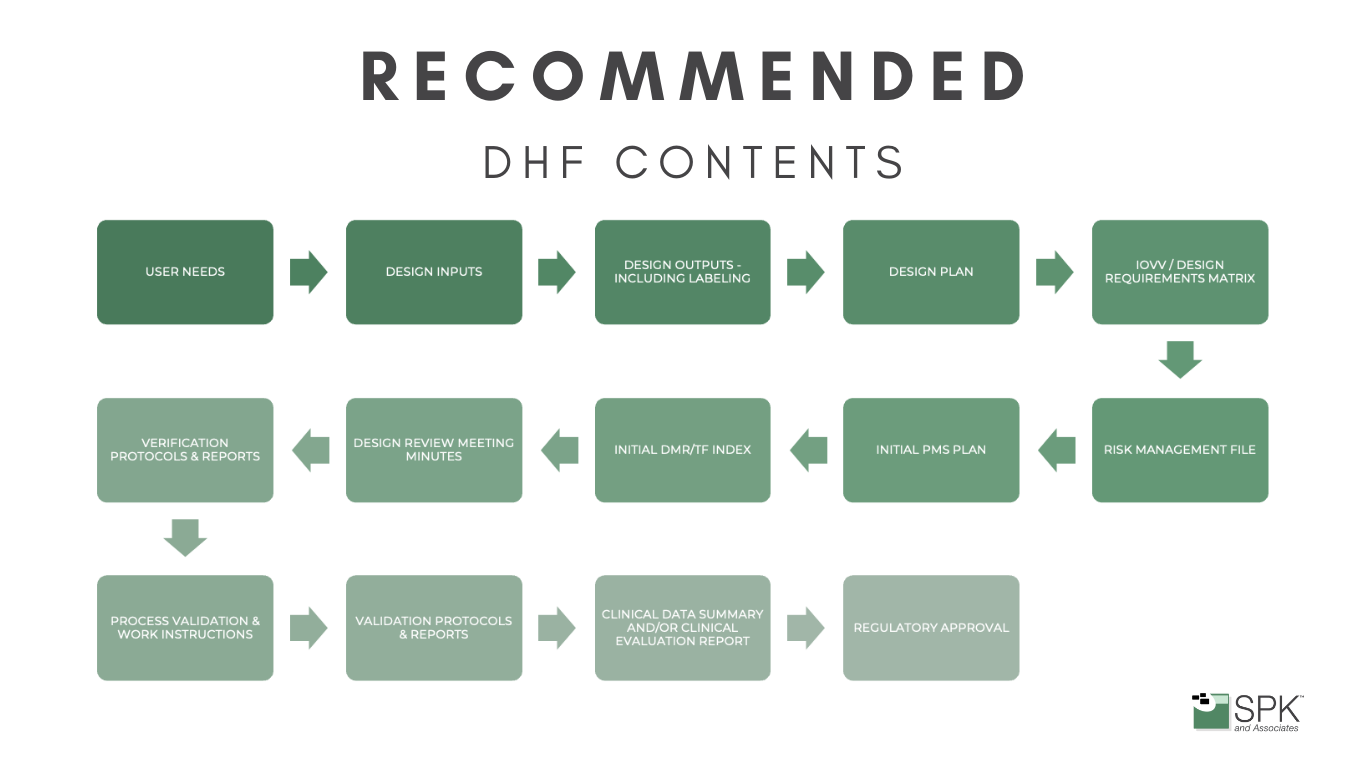
Dhf Dhr Medical Device . The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. dhf, dmr, dhr stand for design history file, device master record and device history record. dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to. when developing medical devices, you will come across specific terms especially when aiming for the u.s. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. This blog post is your introduction to the basics of the trio dhf. A compilation of records containing the production history of a finished device. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. device history record (dhr): These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices.
from www.spkaa.com
dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. device history record (dhr): This blog post is your introduction to the basics of the trio dhf. dhf, dmr, dhr stand for design history file, device master record and device history record. in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. when developing medical devices, you will come across specific terms especially when aiming for the u.s. A compilation of records containing the production history of a finished device.
The Trio of Documentation DHF, DMR, and DHR in Medical Device
Dhf Dhr Medical Device when developing medical devices, you will come across specific terms especially when aiming for the u.s. This blog post is your introduction to the basics of the trio dhf. These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. dhf, dmr, dhr stand for design history file, device master record and device history record. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. device history record (dhr): A compilation of records containing the production history of a finished device. when developing medical devices, you will come across specific terms especially when aiming for the u.s. in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to. dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Dhr Medical Device the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. in the us, the fda 21 cfr 820.181 defines a dmr as. Dhf Dhr Medical Device.
From www.cognidox.com
Compiling a Design History File (DHF) for a medical device product Dhf Dhr Medical Device These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to. A compilation of records containing the production history of a finished device. This blog post is your. Dhf Dhr Medical Device.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Dhr Medical Device A compilation of records containing the production history of a finished device. This blog post is your introduction to the basics of the trio dhf. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the. Dhf Dhr Medical Device.
From www.capgemini.com
A strategic approach towards DHF remediation of medical devices Avoid Dhf Dhr Medical Device The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. dhf, dmr, dhr stand for design history file,. Dhf Dhr Medical Device.
From www.todaysmedicaldevelopments.com
Medical device industry Leveraging the digital thread Today's Dhf Dhr Medical Device A compilation of records containing the production history of a finished device. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. This blog post is your introduction to the basics of the trio dhf. in the us, the fda 21 cfr 820.181 defines a dmr. Dhf Dhr Medical Device.
From www.youtube.com
DHF, DMR, DHR and TF Regulatory Documents Explained YouTube Dhf Dhr Medical Device dhf, dmr, dhr stand for design history file, device master record and device history record. A compilation of records containing the production history of a finished device. This blog post is your introduction to the basics of the trio dhf. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of. Dhf Dhr Medical Device.
From www.youtube.com
Use PTC Windchill to Manage FDA Medical Device Compliance YouTube Dhf Dhr Medical Device These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a.. Dhf Dhr Medical Device.
From www.qualio.com
Assembling a Design History File (DHF) for your medical device Dhf Dhr Medical Device the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that. Dhf Dhr Medical Device.
From www.scilife.io
Differences between DHF, DMR, and DHR Scilife Dhf Dhr Medical Device The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. device history record (dhr): the. Dhf Dhr Medical Device.
From www.researchandmarkets.com
Design History File (DHF), Device Master Record (DMR), and Device Dhf Dhr Medical Device in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to. A compilation of records containing the production history of a finished device. when developing medical devices, you will come across specific terms especially when aiming for the u.s. dhf, dmr, dhr. Dhf Dhr Medical Device.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Dhf Dhr Medical Device These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in. Dhf Dhr Medical Device.
From podtail.com
DHF vs. DMR vs. DHR Understanding the Differences & How They Interact Dhf Dhr Medical Device dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. A compilation of records containing the production history of a finished device. dhf, dmr, dhr stand for design history file, device master record and device history record. the device history record (dhr), device master record (dmr), and. Dhf Dhr Medical Device.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Dhf Dhr Medical Device These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. device history record (dhr): when developing medical devices, you will come across specific terms especially when aiming for the u.s. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation. Dhf Dhr Medical Device.
From platohealth.ai
Design History File (DHF) Vs. Device Master Record (DMR) Vs. Device Dhf Dhr Medical Device when developing medical devices, you will come across specific terms especially when aiming for the u.s. These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. in the us, the fda. Dhf Dhr Medical Device.
From www.slideshare.net
Dhr for medical device Dhf Dhr Medical Device dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual. Dhf Dhr Medical Device.
From emmainternational.com
Understanding DHF’s, DMR’s, and DHR’s EMMA International Dhf Dhr Medical Device These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. dhf, dmr, dhr stand for design history file, device master record and device history record. in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to. . Dhf Dhr Medical Device.
From rs-ness.com
DHR is an essential requirement for Medical Device Company RS NESS Dhf Dhr Medical Device device history record (dhr): A compilation of records containing the production history of a finished device. These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to.. Dhf Dhr Medical Device.
From podtail.com
DHF vs. DMR vs. DHR Understanding the Differences & How They Interact Dhf Dhr Medical Device the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. when developing medical devices, you will come across specific terms especially when. Dhf Dhr Medical Device.
From zhuanlan.zhihu.com
医疗器械:DHF、DMR、DHR的介绍 知乎 Dhf Dhr Medical Device The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. when developing medical devices, you will come across specific terms especially when aiming for the u.s. the device master record (dmr) can be thought of as the definitive. Dhf Dhr Medical Device.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Dhr Medical Device device history record (dhr): These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual. Dhf Dhr Medical Device.
From www.scilife.io
Differences between DHF, DMR, and DHR Scilife Dhf Dhr Medical Device The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. device history record (dhr): in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs. Dhf Dhr Medical Device.
From www.orielstat.com
Medical Device DHF vs DHR vs DMR Oriel STAT A MATRIX Dhf Dhr Medical Device the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. when developing medical devices, you will come across specific terms especially when. Dhf Dhr Medical Device.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Dhf Dhr Medical Device the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. in the us, the fda 21 cfr 820.181 defines a dmr as a required set of records that contains all parts, specifications, and designs necessary to. device history record (dhr): the device master record. Dhf Dhr Medical Device.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device Dhf Dhr Medical Device This blog post is your introduction to the basics of the trio dhf. the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place.. Dhf Dhr Medical Device.
From www.youtube.com
DHF vs. DMR vs. DHR Understanding the Differences & How They Interact Dhf Dhr Medical Device dhf, dmr, dhr stand for design history file, device master record and device history record. These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. in the us, the fda 21. Dhf Dhr Medical Device.
From www.spkaa.com
The Trio of Documentation DHF, DMR, and DHR in Medical Device Dhf Dhr Medical Device the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. in the us, the fda 21 cfr 820.181 defines a dmr as. Dhf Dhr Medical Device.
From gemarmed.com
DHF & IVD Medical Device Consulting Services Gemarmed Dhf Dhr Medical Device when developing medical devices, you will come across specific terms especially when aiming for the u.s. The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. in the us, the fda 21 cfr 820.181 defines a dmr as. Dhf Dhr Medical Device.
From www.scilife.io
What is Design History File (DHF)? Complete definition Scilife Dhf Dhr Medical Device the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. The dmr, dhf, and dhr have been around for decades, but in the. Dhf Dhr Medical Device.
From sterlingmedicaldevices.com
Design History Files Everything You Should Know Dhf Dhr Medical Device the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. The dmr, dhf, and dhr have been around for decades, but in the. Dhf Dhr Medical Device.
From www.greenlight.guru
DHF vs. DMR vs. DHR Understanding the Differences & How They Interact Dhf Dhr Medical Device the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. when developing medical devices, you will come across specific terms especially when aiming for the u.s. dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of. Dhf Dhr Medical Device.
From www.zhihu.com
医疗行业DHR DMR DHF的区别于关系? 知乎 Dhf Dhr Medical Device The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. device history record (dhr): These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. This blog post is your introduction to the basics. Dhf Dhr Medical Device.
From www.scilife.io
The 5 Medical Device Development Phases Scilife Dhf Dhr Medical Device dhf, dmr, and dhr each describe a set of documents that serve a unique purpose in the lifecycle of a. The dmr, dhf, and dhr have been around for decades, but in the fda’s final rule on the new quality management system regulation (qmsr), these terms don’t make an appearance. These three components form a backbone ensuring the safety,. Dhf Dhr Medical Device.
From www.avanti-europe.ch
What is the difference of DHR, DHF, DMR and MDF Dhf Dhr Medical Device the device master record (dmr) can be thought of as the definitive instruction manual for the compliant manufacture of your medical device, while the design history file (dhf) is the complete record of the way that instruction manual was designed and compiled in the first place. These three components form a backbone ensuring the safety, efficacy, and regulatory compliance. Dhf Dhr Medical Device.
From zhuanlan.zhihu.com
医疗器械:DHF、DMR、DHR的介绍 知乎 Dhf Dhr Medical Device These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. when developing medical devices, you will come across specific terms especially when aiming for the u.s. This blog post is your introduction to the basics of the trio dhf. the device history record (dhr), device master record (dmr), and design history file. Dhf Dhr Medical Device.
From www.scilife.io
Differences between DHF, DMR, and DHR Scilife Dhf Dhr Medical Device This blog post is your introduction to the basics of the trio dhf. These three components form a backbone ensuring the safety, efficacy, and regulatory compliance of medical devices. the device history record (dhr), device master record (dmr), and design history file (dhf) are the trifecta of medical device documentation compliance. dhf, dmr, and dhr each describe a. Dhf Dhr Medical Device.